Modulation and control of SENP1 phosphorylated modified compound and SIRT3 SUMOylation modified compound and application of SENP1 phosphorylated modified compound and SIRT3 SUMOylation modified compound
A compound and phosphorylation technology, applied in the field of biomedicine, can solve problems such as unclear mitochondrial metabolic process of SIRT3
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
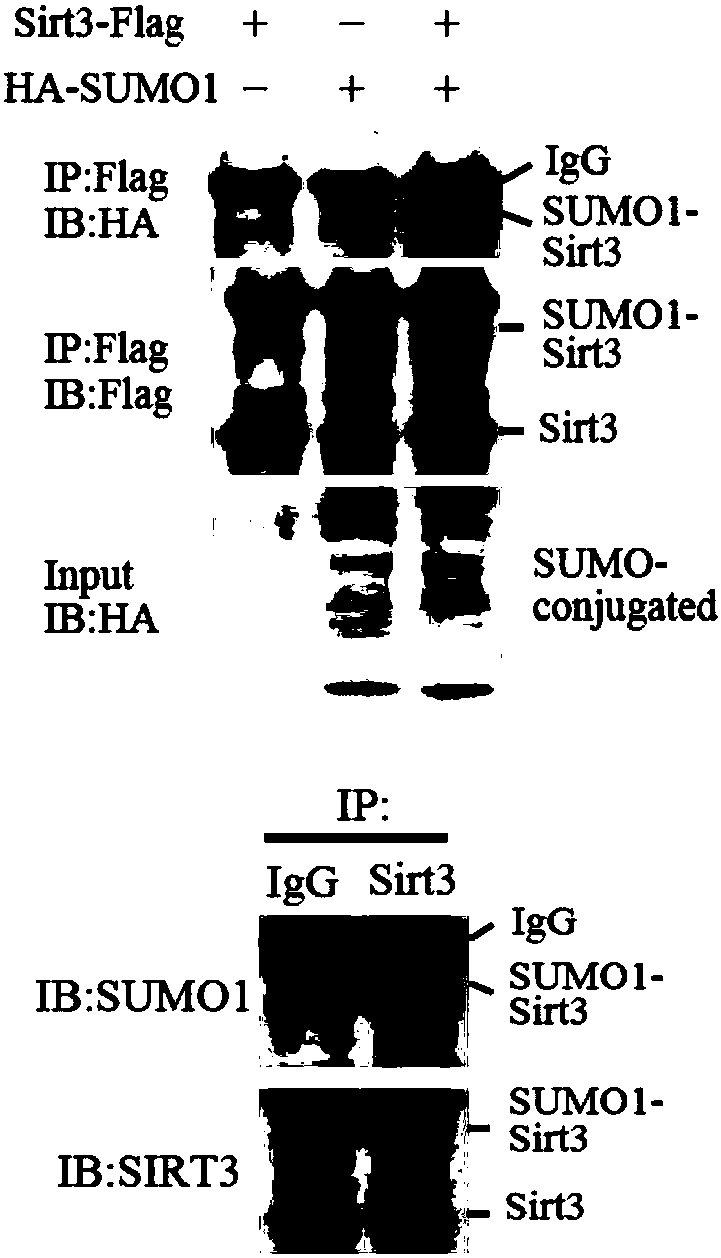
[0048] Example 1 Immunoprecipitation identification of SIRT3 SUMO modification method
[0049] First transfect SIRT3-Flag and HA-SUMO1 into 293T cells to extract mitochondrial components, and then perform immunoprecipitation experiments with M2-Flagaffinity gel (Sigma, A2220) or HA magnetic beads (Thermo, 88836), and use Flag (Sigma, M2) and HA (Sigma, HA-7) antibodies were detected, and a 51kDa SIRT3SUMO modified band was detected ( figure 1 ). Endogenous SIRT3 SUMOylation was detected by extracting mitochondrial fractions from the liver cancer cell line SMMC7721, immunoprecipitating with SIRT3 (Cell Signaling, 5490) antibodies, and then using SIRT3 (Cell Signaling, 5490) and SUMO1 (Abcam, 32058) antibodies, and also detecting SIRT3SUMO modified band to 49kDa size ( figure 1 ). Then, SIRT3-Flag, HA-SUMO1 and RGS-SENP1 plasmids were co-transfected into 293T cells, mitochondrial proteins were extracted and immunoprecipitated with Flag antibody, and the results of Flag and HA...
Embodiment 2
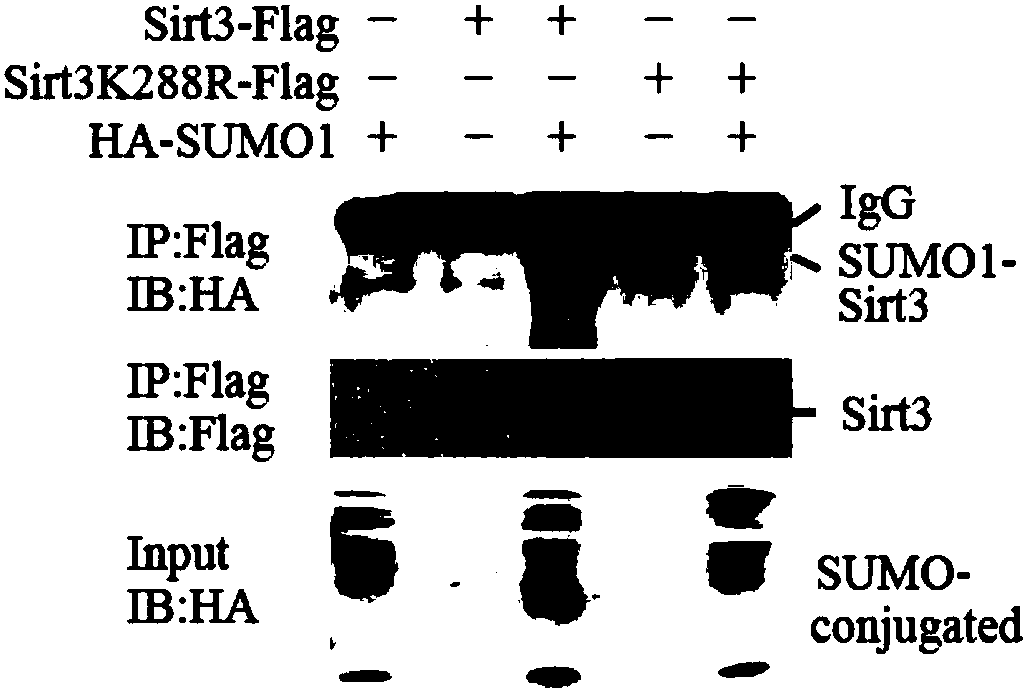
[0071] Embodiment 2 SIRT3SUMO modification site identification method
[0072] Through protein amino acid sequence alignment, the results show that there is a SUMO-modified consensus sequence ψ-K-χ-D around the 288-position lysine of human SIRT3 protein (ψ is a hydrophobic amino acid residue, χ is any amino acid residue ), and this sequence is conserved among different species ( figure 2 ). Use the following pair of primers to mutate the 288th lysine on the SIRT3 plasmid to arginine by PCR method, then co-transfect the SIRT3K288R plasmid and the SUMO1 plasmid into 293T cells, use the SIRT3WT plasmid as a positive control, and pass M2 -Flag affinity gel (Sigma, A2220) or HA magneticbeads (Thermo, 88836) for immunoprecipitation experiments, using Flag (Sigma, M2) and HA (Sigma, HA-7) antibodies for detection, the results show that SIRT3 lysine 288 After mutation, SIRT3 cannot undergo SUMOylation modification, that is, K288 is the SIRT3 SUMOylation modification site ( image ...
Embodiment 3
[0075] Example 3 proves that SIRT3 SUMO modification has an inhibitory effect on the deacetylation level of SIRT3
[0076] Flag-SIRT3WT or Flag-SIRT3K288R expressing cell lines were established by stably transfecting Flag-SIRT3WT or Flag-SIRT3K288R plasmids in endogenous SIRT3 gene silenced liver cancer cells SMMC7721. Immunoprecipitation was carried out by Acetyl-lys (Cell Signaling, 9441) antibody, and then with known SIRT3 deacetylation-modified target protein antibodies such as SOD2 (Abcam, 13534), LCAD (Abcam, 196655), HMGCS2 (Abcam, 137043 ), AceCS2 (Abcam, 66038), the results showed that SIRT3K288R had higher sirtuin activity than wild type ( Figure 5 ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com