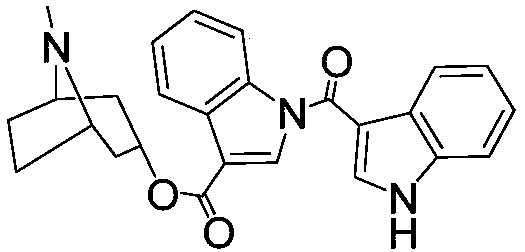
Preparation method of tropisetron bisindole impurities
A technology of tropisetron and bisindole, which is applied in the field of drug synthesis to achieve the effects of mild reaction, good commercial value and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
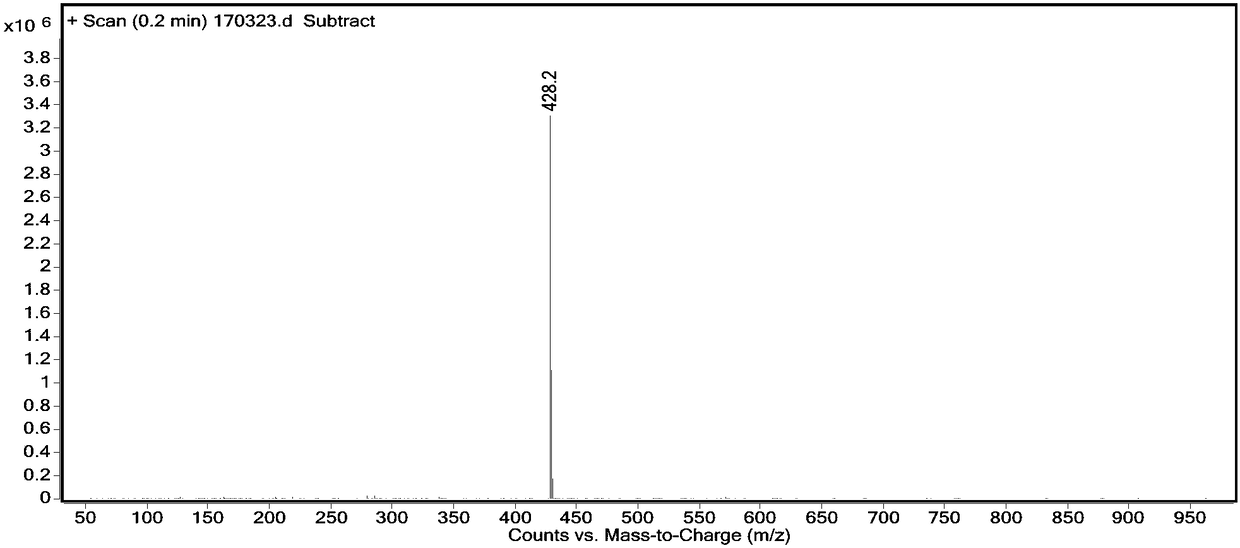
Image
Examples
Embodiment 1
[0023] 3-Indolecarboxylic acid (5.0g, 31mmol) was added to a 100mL reaction flask, 25mL of dichloromethane was added, and oxalyl chloride (5.3mL, 62mmol) was added to react at room temperature for 5h. TLC detected that the raw materials had been reacted (dichloromethane:methanol = 10:1). Post-treatment: filter, remove the filtrate, wash the filter cake twice with dichloromethane, and dry in vacuo to obtain 4.5 g of yellow solid powder with a yield of 81.2%.
Embodiment 2
[0025] 3-Indolecarboxylic acid (5.0 g, 31 mmol) was added to a 100 mL reaction flask, 25 mL of dichloromethane was added, and thionyl chloride (9.2 mL, 124 mmol) was added to react at room temperature for 4 h. It was detected by TLC that the reaction of the starting material had been completed (dichloromethane:methanol=10:1). Post-treatment: filter, remove the filtrate, wash the filter cake twice with dichloromethane, and dry in vacuo to obtain 4.3 g of yellow solid powder with a yield of 78.2%.
Embodiment 3
[0027] 3-Indolecarboxylic acid (5.0 g, 31 mmol) was added to a 100 mL reaction flask, 25 mL of ethyl acetate was added, and oxalyl chloride (15.9 mL, 186 mmol) was added to react at room temperature for 3 h. It was detected by TLC that the reaction of the starting material had been completed (dichloromethane:methanol=10:1). Post-treatment: Distill the solvent off under reduced pressure to obtain a viscous solid, add 10 mL of dichloromethane, beat for 1 hour, filter, remove the filtrate, wash the filter cake twice with dichloromethane, and dry in vacuo to obtain 3.9 g of yellow solid powder. The rate is 70.9%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com