Process Of Manufacture Of Catalyst And Propylene Polymer That Use This Or Copolymer For Propylene Polymerization
A solid catalyst, propylene polymerization technology, applied in the field of polypropylene preparation, can solve the problems of poor catalyst activity and tacticity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] 1. Preparation of solid catalyst
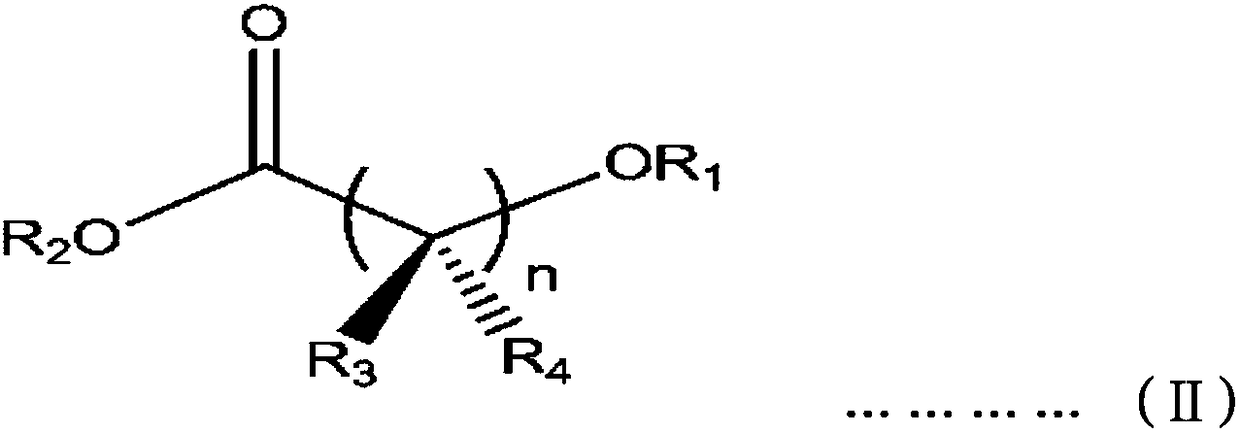
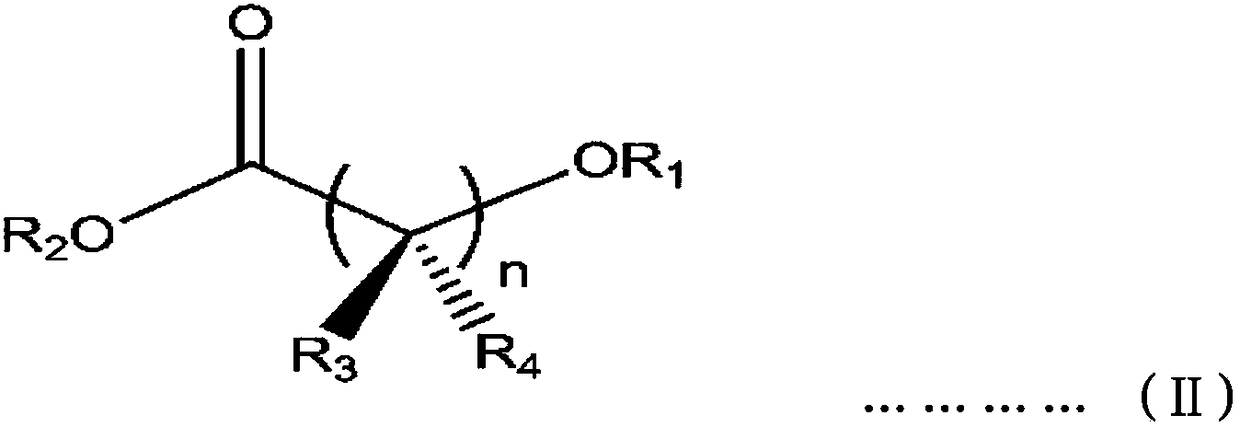
[0053] Add 112ml of toluene and 15g of spherical diethoxymagnesium (average particle size is 20μm, particle distribution index 0.86, apparent density 0.35g / cc), then 20 ml of titanium tetrachloride diluted in 30 ml of toluene was further added thereto, and allowed to react for 1 hour while maintaining the temperature at 10°C. Then, 3.6 g of diisobutyl phthalate and 1.4 g of methyl 4-methoxybutyrate were added thereto while raising the reactor temperature to 100°C. After maintaining the temperature at 100° C. for 2 hours and then lowering to 90° C., stirring was stopped, the supernatant was removed and the resulting product was washed once with an additional 200 ml of toluene. Then 120 ml of toluene and 20 ml of titanium tetrachloride were added thereto, and the temperature was raised to 100°C and maintained for 2 hours, and this process was repeated once. After completion of the aging process, the slurry mixture was washed twice wit...
Embodiment 2
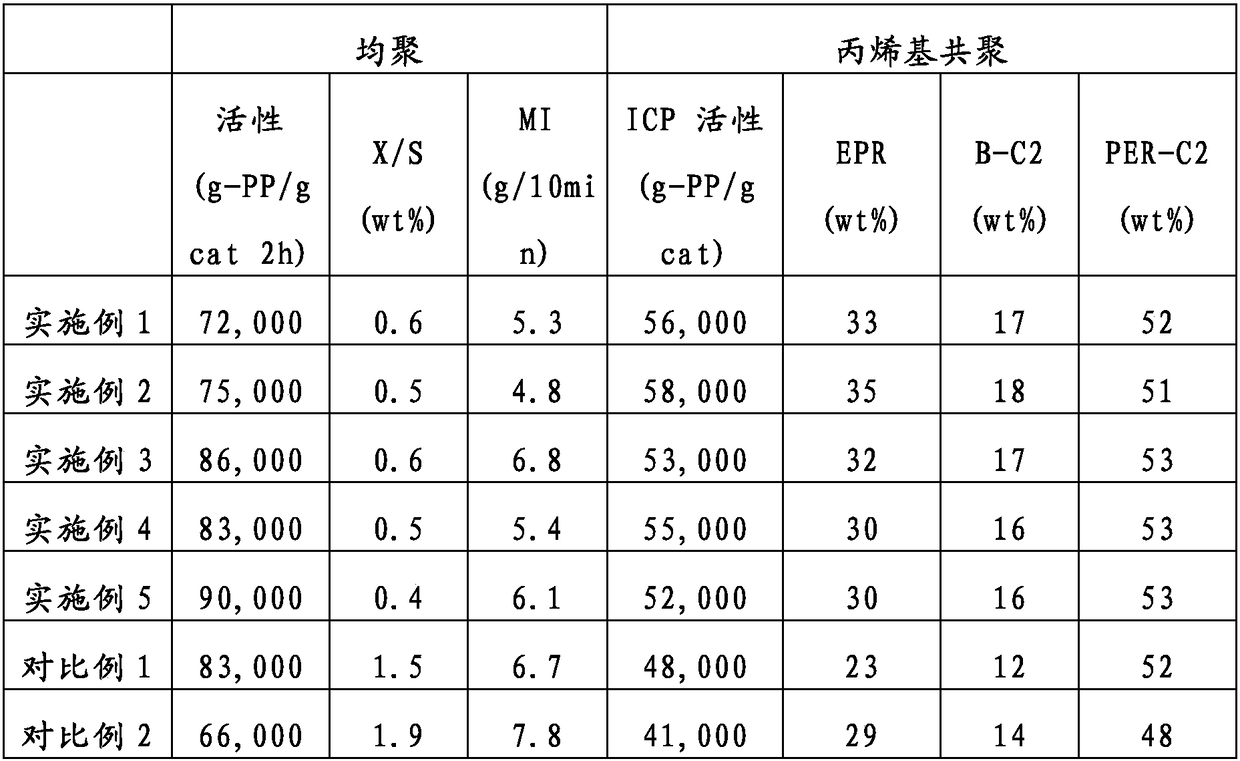
[0068] According to the preparation method of the solid catalyst of Example 1-1, prepare the catalyst, the difference is to add 3.3g diisobutyl phthalate and 2.1g 4-ethoxy ethyl butyrate instead of diisophthalate Mixture of butyl esters and methyl 4-methoxybutyrate. The titanium content in the solid catalyst component was 2.1 wt%. Then, by the same method as in Example 1, polypropylene polymerization and propylene-based copolymerization were carried out, and Table 1 shows the results thereof.
Embodiment 3
[0070] According to the preparation method of the solid catalyst of embodiment 1-1, prepare catalyst, difference is to use the mixture of 4.2g diisobutyl phthalate and 2.8g 5-methoxyvalerate methyl ester to replace phthalic acid Mixture of diisobutyl ester and methyl 4-methoxybutyrate. The titanium content in the solid catalyst component was 2.3 wt%. Then, by the same method as in Example 1, polypropylene polymerization and propylene-based copolymerization were carried out, and Table 1 shows the results thereof.
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
| Apparent density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com