Application of catalyst, and preparation method for disubstituted cyclohexane carboxylate with adjustable cis-trans ratio
A technology of cyclohexane carboxylate and di-substitution, applied in the field of preparation of di-substituted cyclohexane carboxylate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
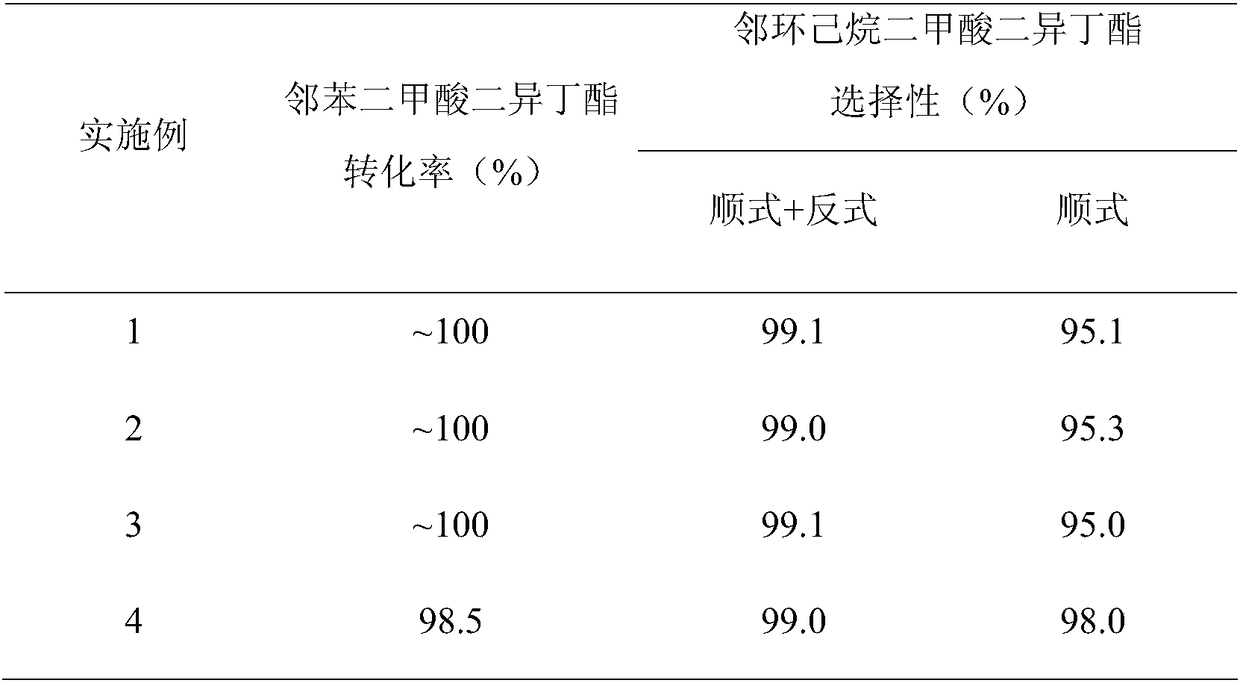
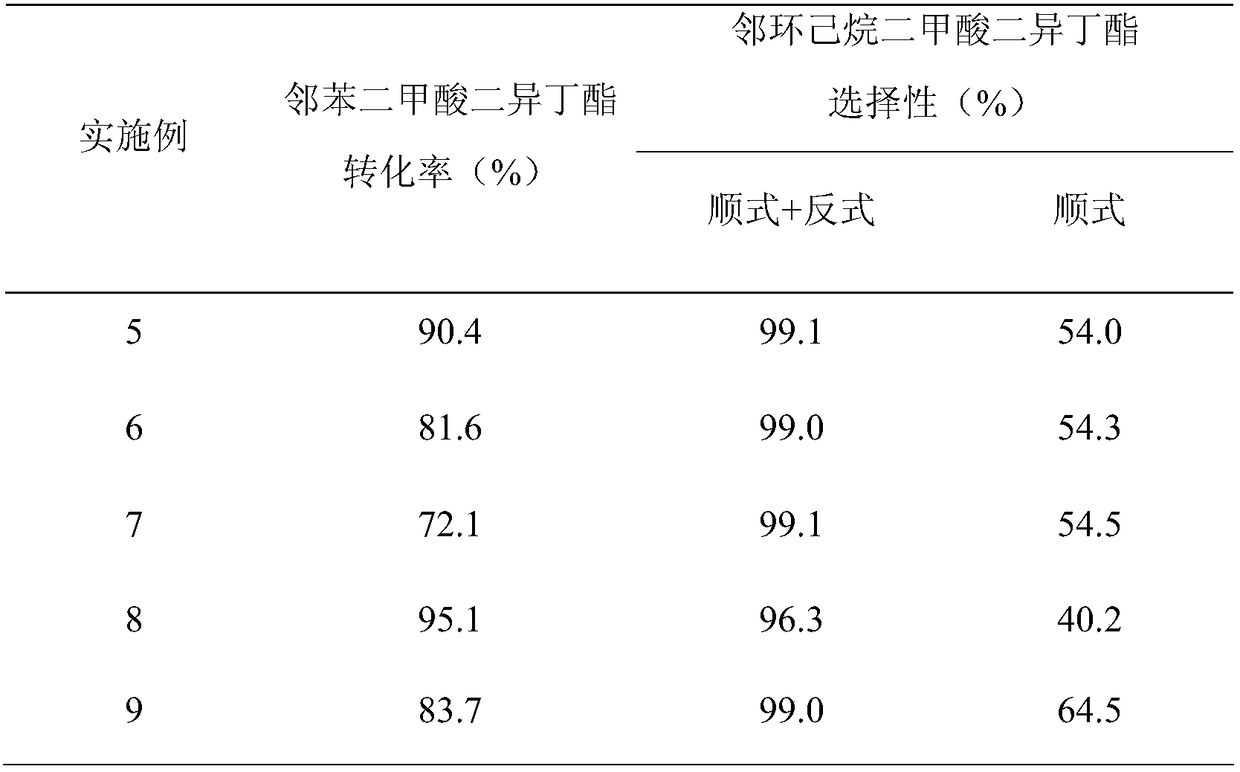
Embodiment 1
[0027] Weigh 0.3298g Pd(NO 3 ) 2 (Pd content 15.16wt%), add 6.5g deionized water, add 4.95g Al after it is completely dissolved 2 O 3 The carrier is stirred uniformly, immersed in an equal volume at 25°C for 12 hours, dried at 95°C and calcined at 400°C for 4 hours to obtain a catalyst precursor. Put the catalyst precursor in 10% H 2 / N 2 (Volume fraction) Activated at 300℃ for 4h in the atmosphere, then 1wt% Pd-Al is obtained 2 O 3 Finished catalyst.
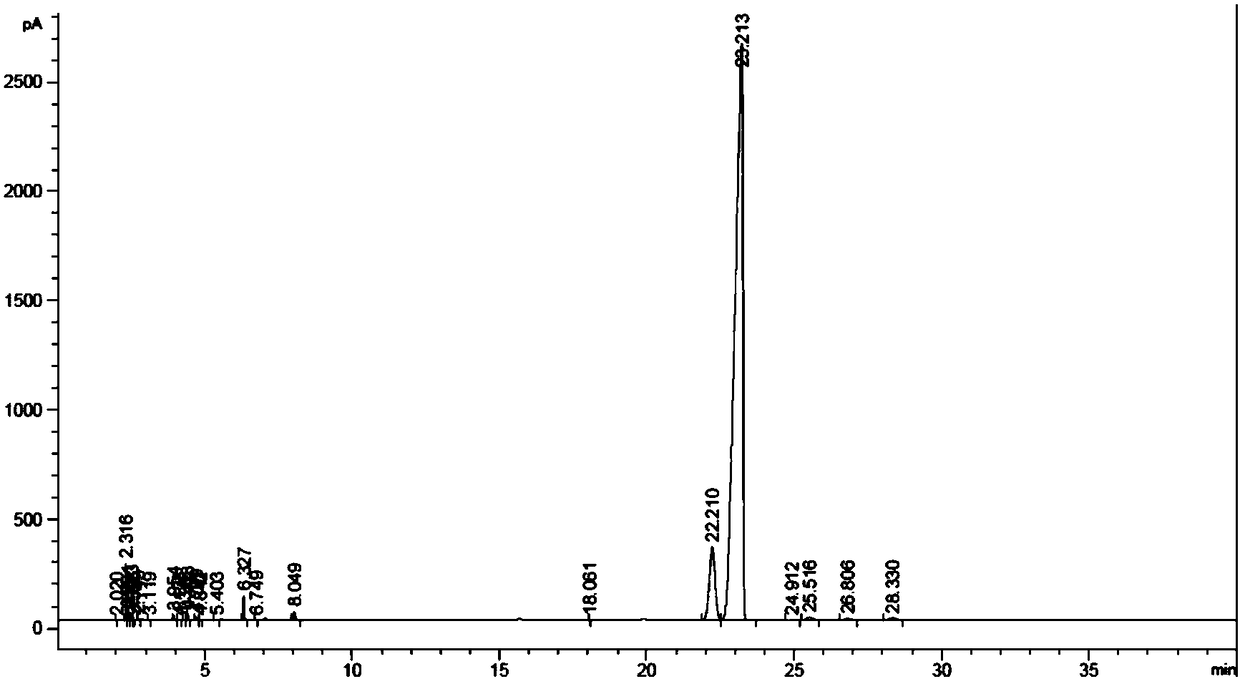
[0028] The hydrogenation reaction of diisobutyl phthalate was carried out in a fixed-bed reactor equipped with a stainless steel tube with an inner diameter of 10 mm. The reaction temperature was 120°C, the hydrogen pressure was 5.0 MPa, and H 2 / Ester molar ratio 125, reactant mass space velocity is 0.6h -1 , Sampling and analysis 24h after the start of the reaction (see figure 1 ), RT=22.2min corresponds to trans-diisobutyl 1,2-cyclohexanedicarboxylate, and RT=23.2min corresponds to cis-1,2-cyclohexanedicarboxylate diisobutyl, ob...
Embodiment 2
[0030] Weigh 1.6490g Pd(NO 3 ) 2 (Pd content 15.16wt%), add 6.2g deionized water, add 4.75g Al after it is completely dissolved 2 O 3 The carrier was stirred uniformly, immersed in an equal volume at 25°C for 12h, dried at 95°C and calcined at 400°C for 4h to obtain a catalyst precursor. Put the catalyst precursor in 10% H 2 / N 2 (Volume Fraction) Activated at 300℃ for 4h in the atmosphere, then 5wt% Pd-Al is obtained 2 O 3 Finished catalyst.
[0031] The hydrogenation reaction of diisobutyl phthalate was carried out in a fixed-bed reactor equipped with a stainless steel tube with an inner diameter of 10 mm. The reaction temperature was 120°C, the hydrogen pressure was 5.0 MPa, and H 2 / Ester molar ratio 125, reactant mass space velocity is 0.6h -1 , Sampling and analysis 24 hours after the start of the reaction, the reaction evaluation results obtained are shown in Table 1.
Embodiment 3
[0033] Weigh 3.2980g Pd(NO 3 ) 2 (Pd content 15.16wt%), add 5.9g deionized water, and add 4.50g Al after it is completely dissolved 2 O 3 The carrier was stirred uniformly, immersed in an equal volume at 25°C for 12h, dried at 95°C and calcined at 400°C for 4h to obtain a catalyst precursor. Put the catalyst precursor in 10% H 2 / N 2 (Volume fraction) Activated at 300℃ for 4h in the atmosphere, then 10wt% Pd-Al is obtained 2 O 3 Finished catalyst.
[0034] The hydrogenation reaction of diisobutyl phthalate was carried out in a fixed-bed reactor equipped with a stainless steel tube with an inner diameter of 10 mm. The reaction temperature was 120°C, the hydrogen pressure was 5.0 MPa, and H 2 / Ester molar ratio 125, reactant mass space velocity is 0.6h -1 , Sampling and analysis 24 hours after the start of the reaction, the reaction evaluation results obtained are shown in Table 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com