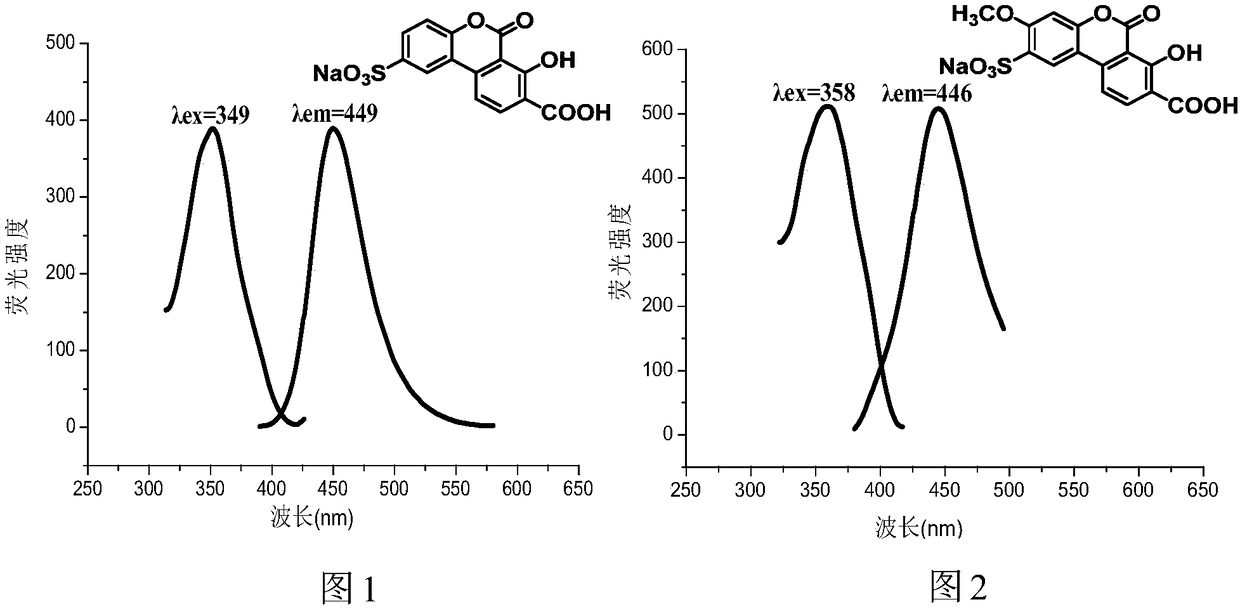
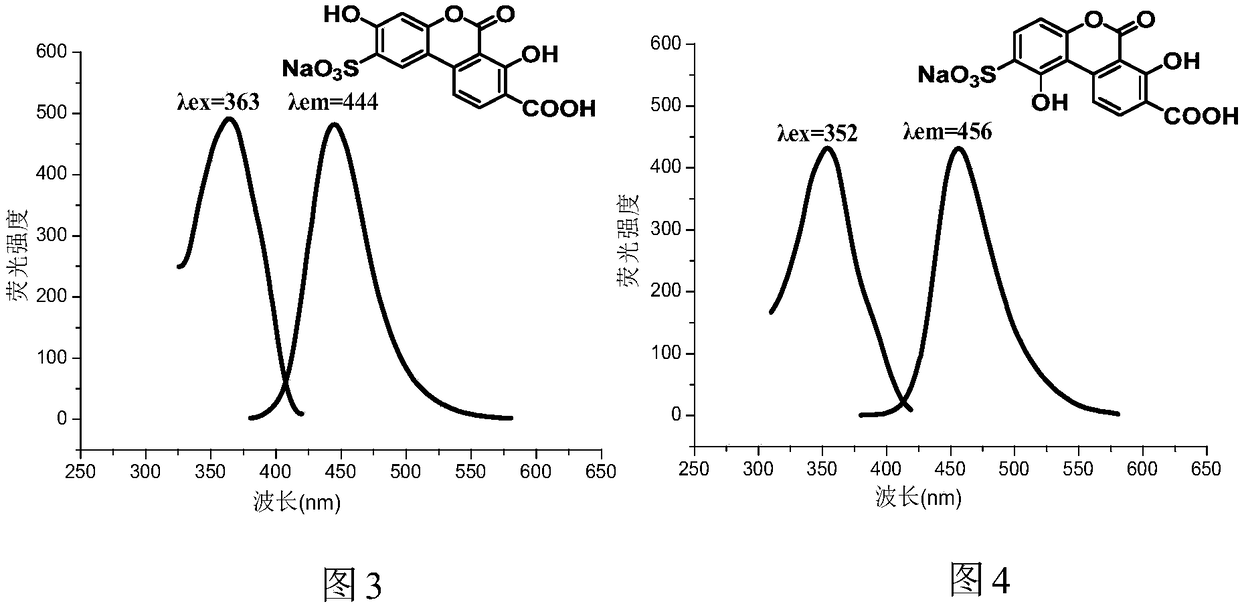
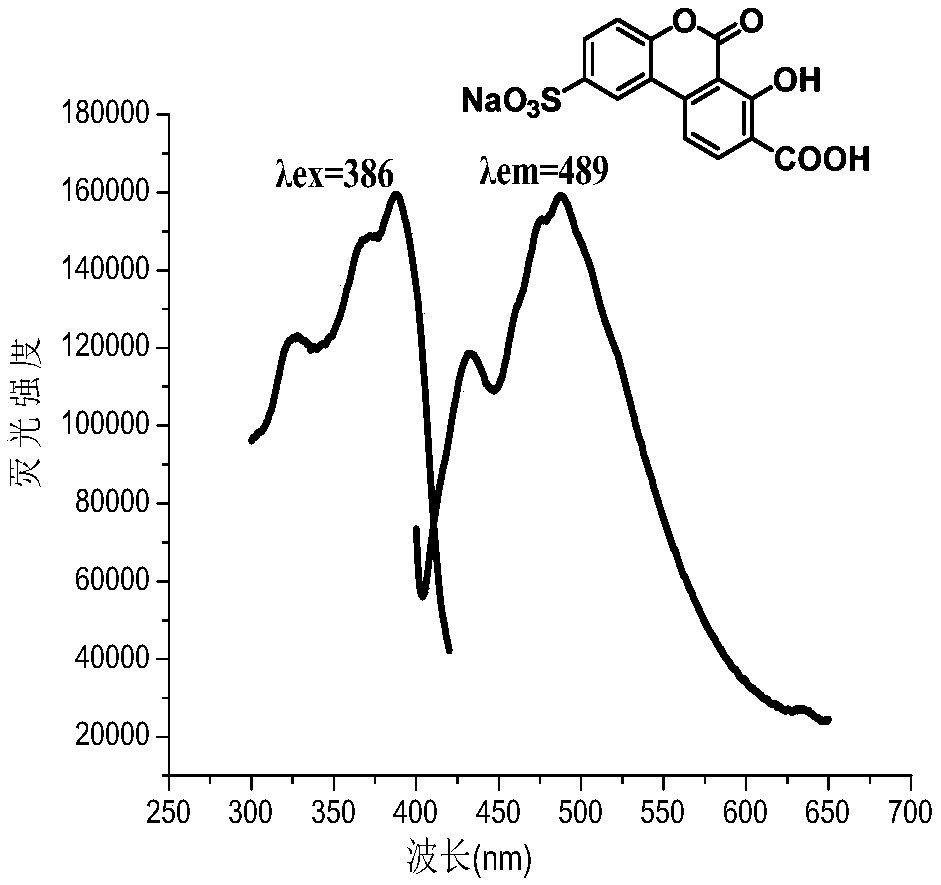
Water-soluble carboxyl-containing hydroxyl benzo coumarin sodium sulfonate and preparation method and application thereof
A technology of sodium coumarin sulfonate and benzocoumarin carboxylic acid, applied in chemical instruments and methods, fluorescence/phosphorescence, luminescent materials, etc. Limitation and other issues, to achieve the effect of low production cost, easy operation and high utilization rate of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Preparation of compounds a~d
[0038] Add 0.5 g of 7-hydroxy-6-oxo-6H-benzo[c]chromene-8-carboxylic acid into a 25 mL reactor, and slowly add 2.0 mL of sulfuric acid with a mass concentration of 98%, dropwise After completion, react at 60°C for 2 hours. After the reaction is complete, cool it down, pour it into saturated NaCl aqueous solution, and precipitate a light yellow precipitate. After filtering the precipitate, salting out and purifying, the pure product of compound a is obtained.
[0039] In this example, the same mass of 7-hydroxy-3-methoxy-6-oxo-6H-benzo[c]chromene-8-carboxylic acid, 3,7-dihydroxy-6- Oxo-6H-benzo[c]chromene-8-carboxylic acid, 1,7-dihydroxy-6-oxo-6H-benzo[c]chromene-8-carboxylic acid were replaced by For 7-hydroxy-6-oxo-6H-benzo[c]chromene-8-carboxylic acid in Example 1, other steps were the same as in Example 1 to obtain compounds b, c, and d sequentially.
[0040] Table 1 Substituents, chemical names and yields of compounds a~d
[0041] ...
Embodiment 2
[0054] In this example, the amount of sulfuric acid with a mass concentration of 98% was 3.0 mL. Other steps were the same as in Example 1 to obtain compounds a to d. The yields of each compound are shown in Table 3.
[0055] The productive rate of compound a~d when table 3 solid-liquid ratio is 1g:6mL
[0056] compound a
Embodiment 3
[0058] In this example, the amount of sulfuric acid with a mass concentration of 98% was 1.5 mL. Other steps were the same as in Example 1 to obtain compounds a to d. The yields of each compound are shown in Table 4.
[0059] The productive rate of compound a~d when table 4 solid-liquid ratio is 1g:3mL
[0060] compound a
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com