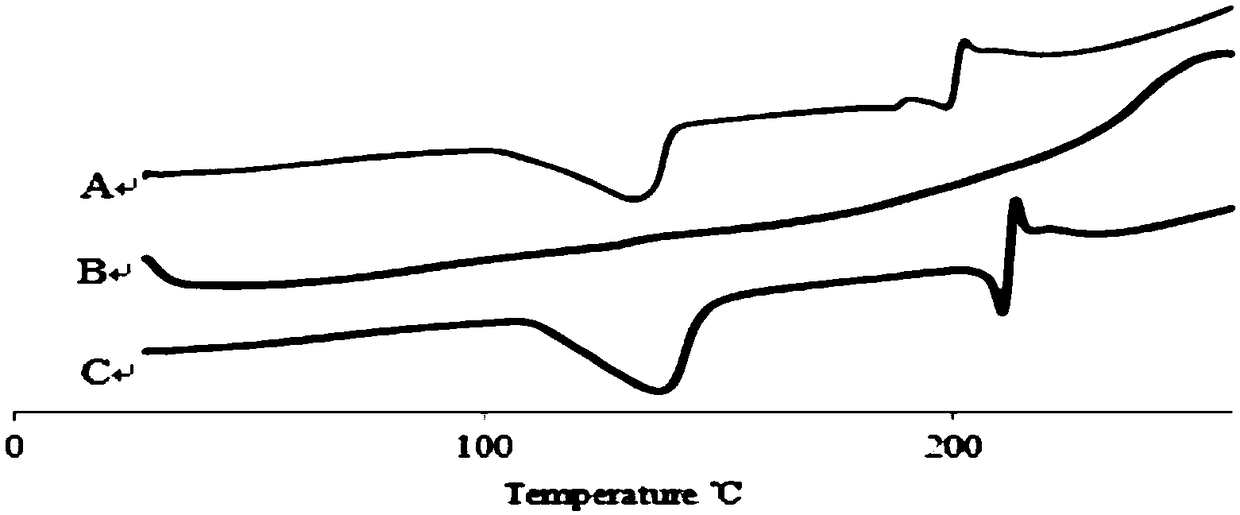
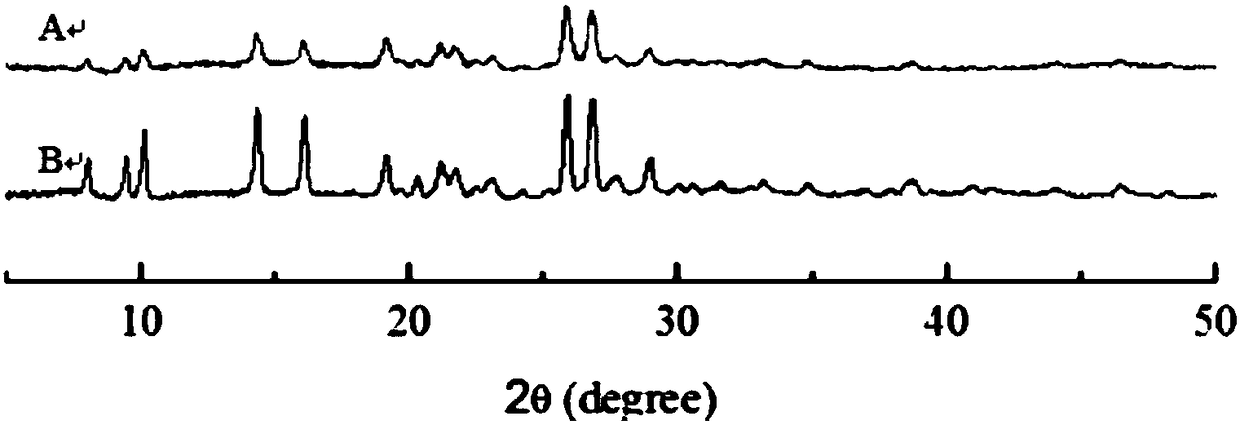
Breviscapine nano particles and preparation method thereof
A technology of breviscapine and nanoparticles, which is applied in the direction of pharmaceutical formulas, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., can solve the problems of short half-life, low oral bioavailability, and poor solubility of scutellarin and other problems, to achieve the effect of improving dissolution rate, stable product quality and good reproducibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0028] The embodiment of the present invention also provides a preparation method of scutellarin nanoparticles, comprising the following steps:
[0029] Mix breviscapine and stabilizer according to the corresponding ratio to obtain a suspension, then pour the suspension into a media grinder, and then add grinding media for grinding.
[0030] The grinding media is a zirconium compound, preferably zirconium oxide. Zirconia has high hardness and strong wear resistance. It is suitable for ultra-fine grinding and dispersion of "zero pollution" and high-viscosity samples. The suspension is a sample with high viscosity. Zirconia can be used for samples. Grind finely.
[0031] Further, the particle size of the grinding medium is 0.4-0.6 mm. The smaller the diameter of the grinding beads, the larger the surface area, the more points of contact with the drug, and the greater the frequency of collisions between the drug and the grinding beads, but the grinding beads with small diameter...
Embodiment 1
[0040] This embodiment provides a scutellarin nanoparticle, which is mainly made of scutellarin and a stabilizer, and the mass of scutellarin accounts for 25% of the total suspension of scutellarin and the stabilizer; wherein, The stabilizer is Tween 80; the mass of the stabilizer accounts for 15% of the mass of breviscapine.
[0041] This embodiment also provides a preparation method of scutellarin nanoparticles, comprising the following steps:
[0042] Weigh 7.5g of breviscapine medicine, place it in 22.5ml of aqueous solution containing 1.125g of Tween 80, stir and suspend to obtain a suspension. Then pour the suspension into the grinding chamber of the media mill, add 450g of zirconia beads with a diameter of 0.6mm, install the machine, turn on the circulating cooling water, and run at a speed of 1400r / min for 1h. Finally, freeze-dry, take the suspension and divide it into 25ml vials, place it at -40°C for 5 hours, and heat it up to -20°C in the first step of the sublimat...
Embodiment 2
[0044] This embodiment provides a scutellarin nanoparticle, which is mainly made of scutellarin and a stabilizer, and the mass of scutellarin accounts for 22% of the total suspension of scutellarin and the stabilizer; wherein, The stabilizer is sodium lauryl sulfate; the mass of the stabilizer accounts for 18% of the mass of breviscapine.
[0045] This embodiment also provides a preparation method of scutellarin nanoparticles, comprising the following steps:
[0046] Weigh 7g of scutellarin, place it in 31.9ml of aqueous solution containing 1.26g of sodium lauryl sulfate, stir and suspend to obtain a suspension. Then pour the suspension into the grinding chamber of the media mill, add 445g of zirconia beads with a diameter of 0.4mm, install the machine, turn on the circulating cooling water, and run at a speed of 1300r / min for 1.5h. Finally, freeze-dry, take the suspension and divide it into 25ml vials, place it at -50°C for 5.5 hours, and heat it up to -17°C in the first ste...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com