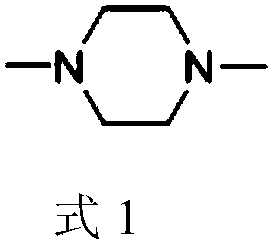
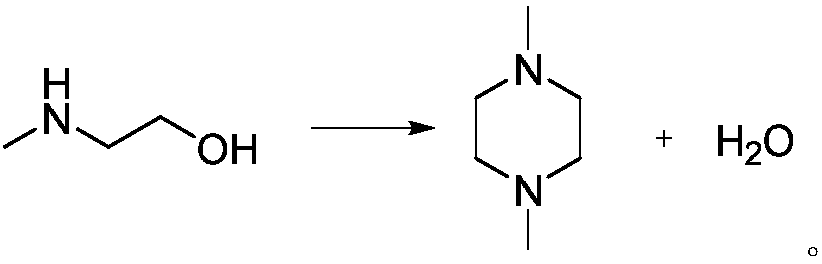
Method of synthesizing 1,4-dimethylpiperazine and catalyst used
A technology of dimethylpiperazine and a supported catalyst is applied in the field of synthesizing 1,4-dimethylpiperazine and the catalyst used, and can solve the problem of low atom utilization rate, low economic value, and by-product methylpiperazine. and other problems, to achieve the effect of low cost, suitable reaction conditions and good activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
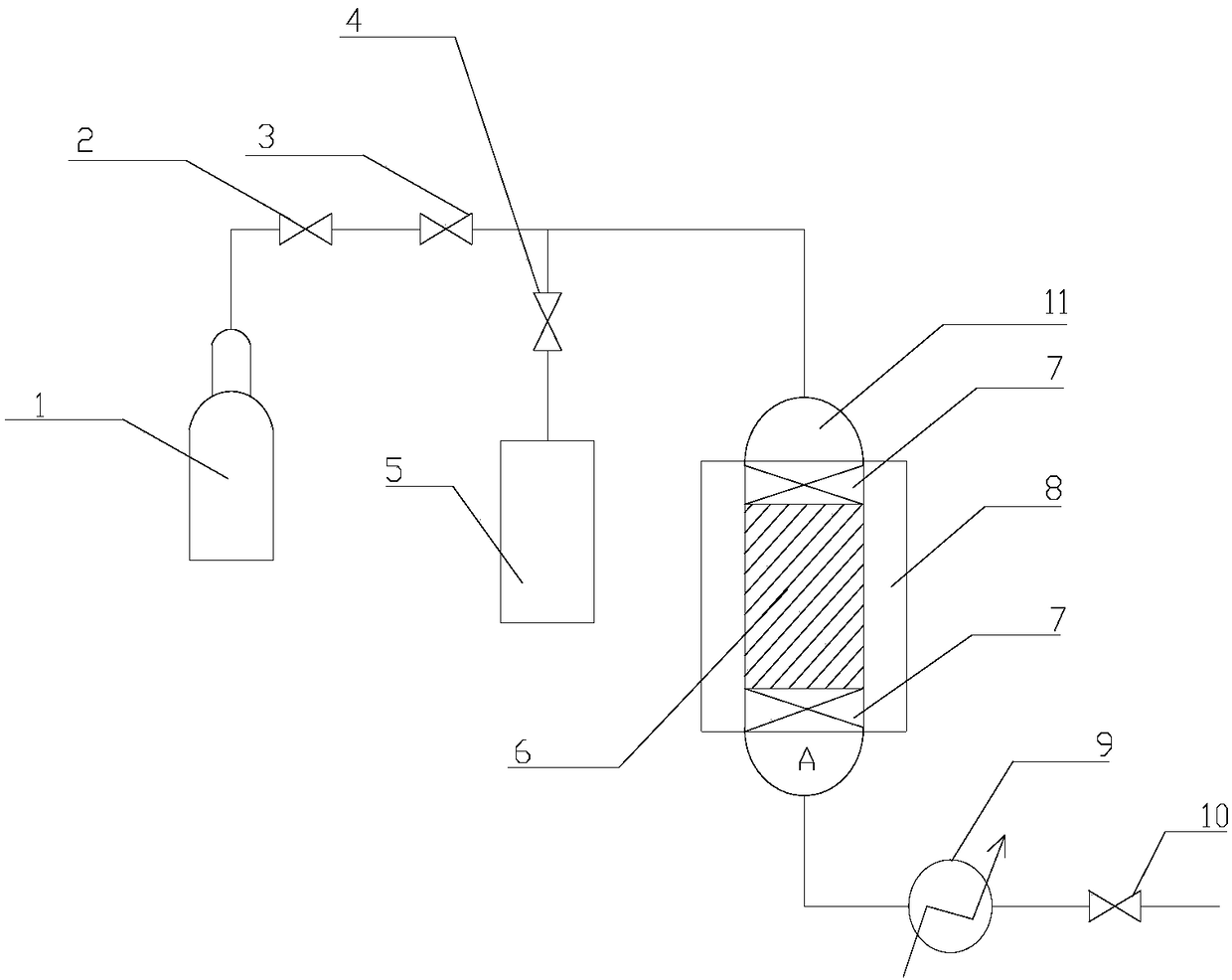
[0035] Embodiment 1, a kind of fixed bed reactor, such as figure 1 As shown, it includes a hydrogen cylinder 1, a sample pump 5 and a fixed bed reactor A, and the fixed bed reactor A includes a vaporization chamber 11 and a catalyst bed 6 arranged in sequence from top to bottom, and the middle part of the catalyst bed 6 A catalyst is arranged, and an inert filler 7 is respectively arranged at the upper and lower ends of the catalyst. The outer surface of the catalyst bed 6 is placed in a molten salt bath 8 which is responsible for regulating the reaction temperature in the catalyst bed 6 .
[0036] The outlet of the hydrogen cylinder 1 and the outlet of the sampling pump 5 are connected together to communicate with the top of the vaporization chamber 11; A valve 4 is set between the outlet of 5 and the confluence point; the bottom outlet of the catalyst bed 6 is connected to the condenser 9 and the valve 10 in sequence.
Embodiment 2
[0037] Embodiment 2: the preparation of supported catalyst
[0038] 1) Roasting: Weigh 1000g of spherical γ-Al with a particle diameter of 2-3mm 2 o 3 , calcined at 700°C for 6h to obtain calcined γ-Al 2 o 3 .
[0039] 2) Solution configuration: Weigh 770.5g of copper nitrate trihydrate and 796.3g of nickel nitrate hexahydrate and dissolve them in water, and set the volume to 2500ml to obtain a mixed solution.
[0040] 3), impregnation: the roasted γ-Al obtained in step 1) 2 o 3 Immerse in the solution prepared in step 2), filter after immersing for 24 hours.
[0041] 4), drying: drying the filter cake obtained in step 3) at 100° C. for 8 hours.
[0042] 5) Roasting: the dried product in step 4) is calcined at 500° C. for 8 hours.
[0043] 6), immerse the product of step 5) in the remaining mixed solution after step 3) again, repeat the above steps 3) to 5); repeat 5 times in total, at this time, the mixed solution obtained in step 2) is completely After the absorptio...
Embodiment 3
[0046] Embodiment 3, a kind of method for synthesizing 1,4-dimethylpiperazine, carry out following steps successively:
[0047] 1), get loaded catalyst 300ml and put it in the catalyst bed 6 of the fixed bed reactor as described in embodiment 1,
[0048] First close the valve 4, open the pressure reducing valve 2 and the valve 10, the hydrogen in the hydrogen cylinder 1 flows into the catalyst bed 6, the supported catalyst is activated under the action of hydrogen, the activation temperature is 200°C, the hydrogen pressure is 2MPa, after 24 hours of activation No liquid flows from valve 10, indicating that the supported catalyst has been activated.
[0049] Remarks: The above-mentioned hydrogen pressure is controlled by adjusting the pressure reducing valve 2, the flow meter 3 is used to observe and control the hydrogen flow rate, the sampling pump 5 and valve 4 are closed during activation, the molten salt bath 8 is responsible for controlling the activation temperature, and ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com