Eugenol ester analogue and its preparation method and insecticide
A technology for eugenol esters and analogs, applied to eugenol ester analogs and their application fields, can solve the problems of poor ester solubility, limited application scope, low hydrophobicity, etc. Effect, simple preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
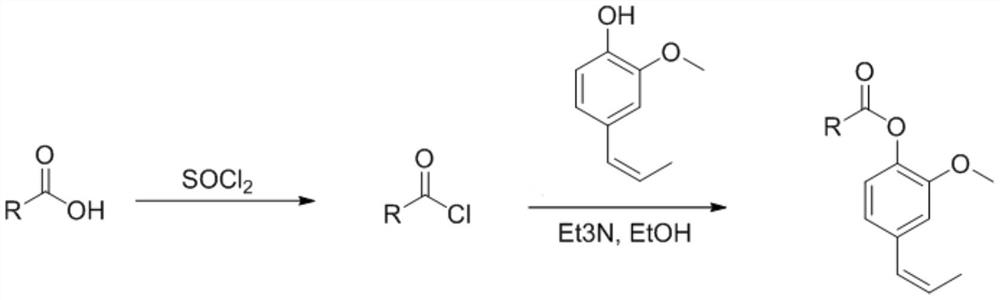
[0027] The present invention also provides a kind of preparation method of described eugenol ester analogue, comprises the following steps:
[0028] (1) peracyl chlorination of the substituted carboxylic acid to obtain an intermediate product;
[0029] (2) reacting the intermediate product and eugenol in the presence of an acid-binding agent and an organic solvent to obtain the eugenol ester analogue;
[0030] Wherein, the substituent in the substituted carboxylic acid is selected from one of C1-C10 alkyl, C1-C10 alkenyl, aryl, furyl and imidazolyl.
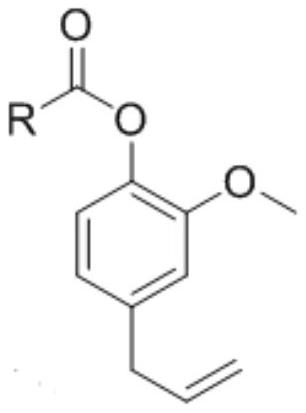
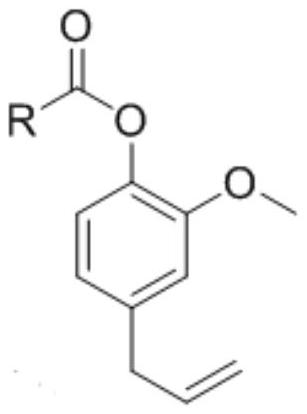
[0031] According to the present invention, the synthetic route of described eugenol ester analog is as follows:
[0032]
[0033] According to the present invention, under preferred conditions, the peracylchlorination process of the substituted carboxylic acid is as follows: uniformly mix the substituted carboxylic acid and thionyl chloride in an organic solvent, heat and reflux at 60°C for 5h, and then cool to 25°C , under ...
Embodiment 1
[0044] Preparation of phenyl 4-propenyl-2-methoxybenzoate
[0045] Mix 10ml of thionyl chloride with 1.00g of benzoic acid (8.19mmol) in dichloromethane evenly, then heat to reflux at 60°C for 5h, then cool to 25°C, at a temperature of 45°C and a pressure of 760mmHg Excess thionyl chloride and dichloromethane were distilled off under reduced pressure to obtain intermediate product 1 as white powder.
[0046] The intermediate product 1 (8.19mmol), 10ml of dichloromethane, 1.34g (8.19mmol) of eugenol, 1ml of triethylamine were stirred at 25°C for 3h, concentrated under reduced pressure, and column chromatography (V 洗脱液 :V 乙酸乙酯 :V 石油醚 =1:3:2) 1.97g of white solid (I1) was obtained with a yield of 90%. The results are shown in Table 1.
[0047] 1 H NMRδ: 2.91(s,3H,CH 3 ), 3.38~3.40 (d, 2H, J=6.00Hz, CH 2 ),3.83(s,1H,OCH 3),5.08~5.31(m,1H,ArH),6.76~6.80(m,2H,ArH),6.94~6.97(m,1H,ArH).
Embodiment 2
[0049] Preparation of methyl 4-propenyl-2-methoxyphenylfuran-2-carboxylate (I2)
[0050] Mix 10ml of thionyl chloride with 1.00g of furancarboxylic acid (8.92mmol) in dichloromethane evenly, then heat to reflux at 60°C for 1h, then cool to 25°C, at a temperature of 45°C and a pressure of 760mmHg The excess thionyl chloride and dichloromethane were distilled off under reduced pressure to obtain white powder intermediate product 2, and the results are shown in Table 1.
[0051] The intermediate product 2 was stirred with 10ml of dichloromethane, 1.52g (8.92mmol) of eugenol, and 1ml of triethylamine in a three-necked flask at 25°C for 4h, then concentrated under reduced pressure, and column chromatography (V 洗脱液 :V 乙酸乙酯 :V 石油醚 =1:1:2) to obtain 1.97g white powder (I2), yield 66%.
[0052] 1 H NMRδ: 3.40~3.42 (d, 2H, J=6.00Hz, CH 2 ),3.82(s,1H,OCH 3 ), 5.09~5.16(m,2H,CH), 5.98~6.00(m,2H,CH), 6.80~6.84(m,2H,CH), 7.06~7.08(m,2H,CH), 7.39~7.40( m, 1H, ArH), 7.67-7.68 (m, 1H, A...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com