Method for synthesizing 4-chloro-3-trifluoromethylaniline by micro-channel reactor
A micro-channel reactor, trifluoromethylaniline technology, applied in chemical instruments and methods, preparation of organic compounds, preparation of nitro compounds, etc., can solve the problems of low number of times of catalyst recovery, long reaction time, violent explosion, etc. , to achieve the effect of controlling the formation of high temperature degradation impurities, long reaction time and short reaction time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] The method that embodiment 1. microchannel reactor synthesizes 4-chloro-3-trifluoromethylaniline.
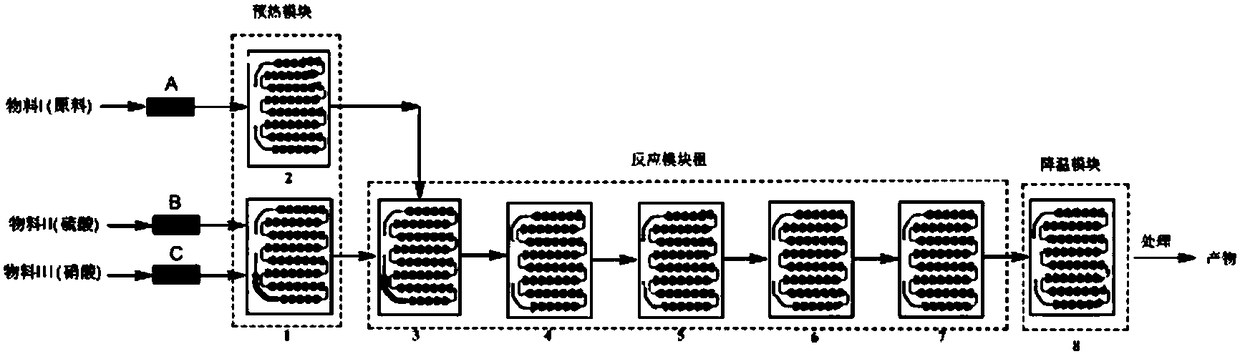
[0046] 1) 320g raw material ortho-chlorobenzotrifluoride is used as material I, enters preheating module 2, concentrated sulfuric acid is used as material II, concentrated nitric acid is used as material III, material II and material III enter preheating module 1 together, carry out mixing and preheating, Then materials I, II, and III carry out nitration reaction in the reaction module group, such as figure 2 As shown, the flow rate of the flow pump is adjusted so that the flow rate of material I is 10ml / min, the flow rate of material II is 15ml / min, the flow rate of material III is 6ml / min, and the reaction temperature is 30°C. 3 The molar ratio of concentrated nitric acid to concentrated sulfuric acid is 1:1.5, the mass ratio of concentrated nitric acid to concentrated sulfuric acid is 1:4.0, the residence time of the reaction is 80s, the temperature of the cooling mod...
Embodiment 2
[0048] Embodiment 2. The method for the synthesis of 4-chloro-3-trifluoromethylaniline in a microchannel reactor.
[0049] 1) 400g raw material ortho-chlorobenzotrifluoride is used as material I, enters preheating module 2, concentrated sulfuric acid is used as material II, concentrated nitric acid is used as material III, material II and material III enter preheating module 1 together, mix and preheat, Then materials I, II, and III carry out nitration reaction in the reaction module group, such as figure 2 As shown, the flow rate of the flow pump is adjusted so that the flow rate of material I is 15ml / min, the flow rate of material II is 18ml / min, the flow rate of material III is 11.5ml / min, and the reaction temperature is 60°C. 3 The molar ratio of concentrated nitric acid to concentrated sulfuric acid is 1:4.0, the residence time of the reaction is 55 seconds, the temperature of the cooling module is 25°C, and the reaction liquid flowing out from the outlet of the cooling ...
Embodiment 3
[0051] The method of embodiment 3. microchannel reactor synthesis 4-chloro-3-trifluoromethylaniline.
[0052] 1) 380g raw material o-chlorobenzotrifluoride is used as material I, enters preheating module 2 for preheating, concentrated sulfuric acid is used as material II, concentrated nitric acid is used as material III, material II and material III enter preheating module 1 together, mix and Preheating, then materials I, II, III carry out nitration reaction in the reaction module group, such as figure 2 As shown, the flow rate of the flow pump is adjusted so that the flow rate of material I is 30ml / min, the flow rate of material II is 40ml / min, the flow rate of material III is 22.5ml / min, and the reaction temperature is 50°C. 3 The molar ratio of concentrated nitric acid to concentrated sulfuric acid is 1:3, the residence time of the reaction is 27 seconds, the temperature of the cooling module is 30°C, and the reaction liquid flowing out from the outlet of the cooling modul...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com