Carboxylic acid derivatization beta-carboline and preparation method thereof
A derivatization and carboline technology, which is applied in the fields of chemistry and medicine, can solve problems such as insufficient structural diversity, and achieve the effects of wide application range of substrates, strong functional group tolerance, and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0020] In order to further understand the present invention, the preferred embodiments of the present invention are described below in conjunction with the examples, but it should be understood that these descriptions are only to further illustrate the features and advantages of the present invention, rather than limiting the claims of the present invention.
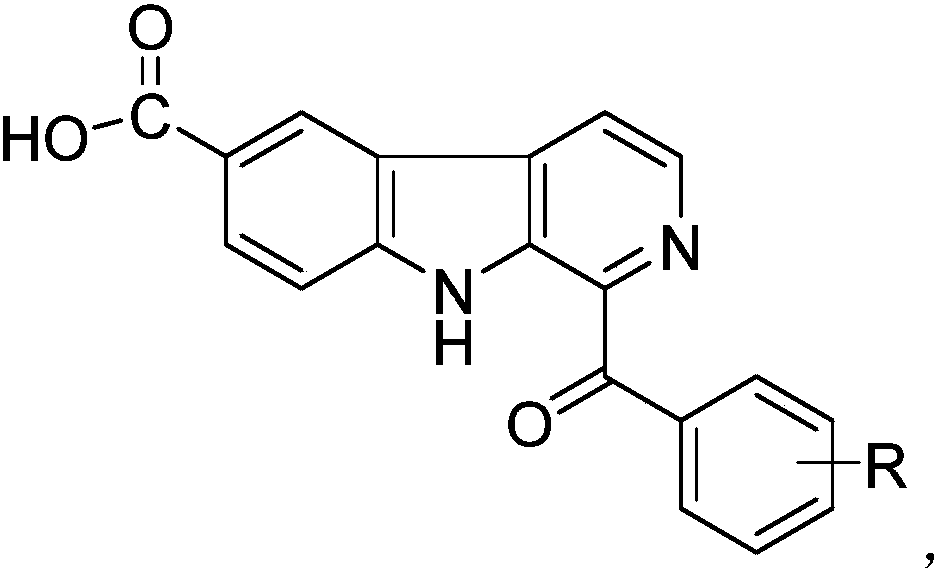
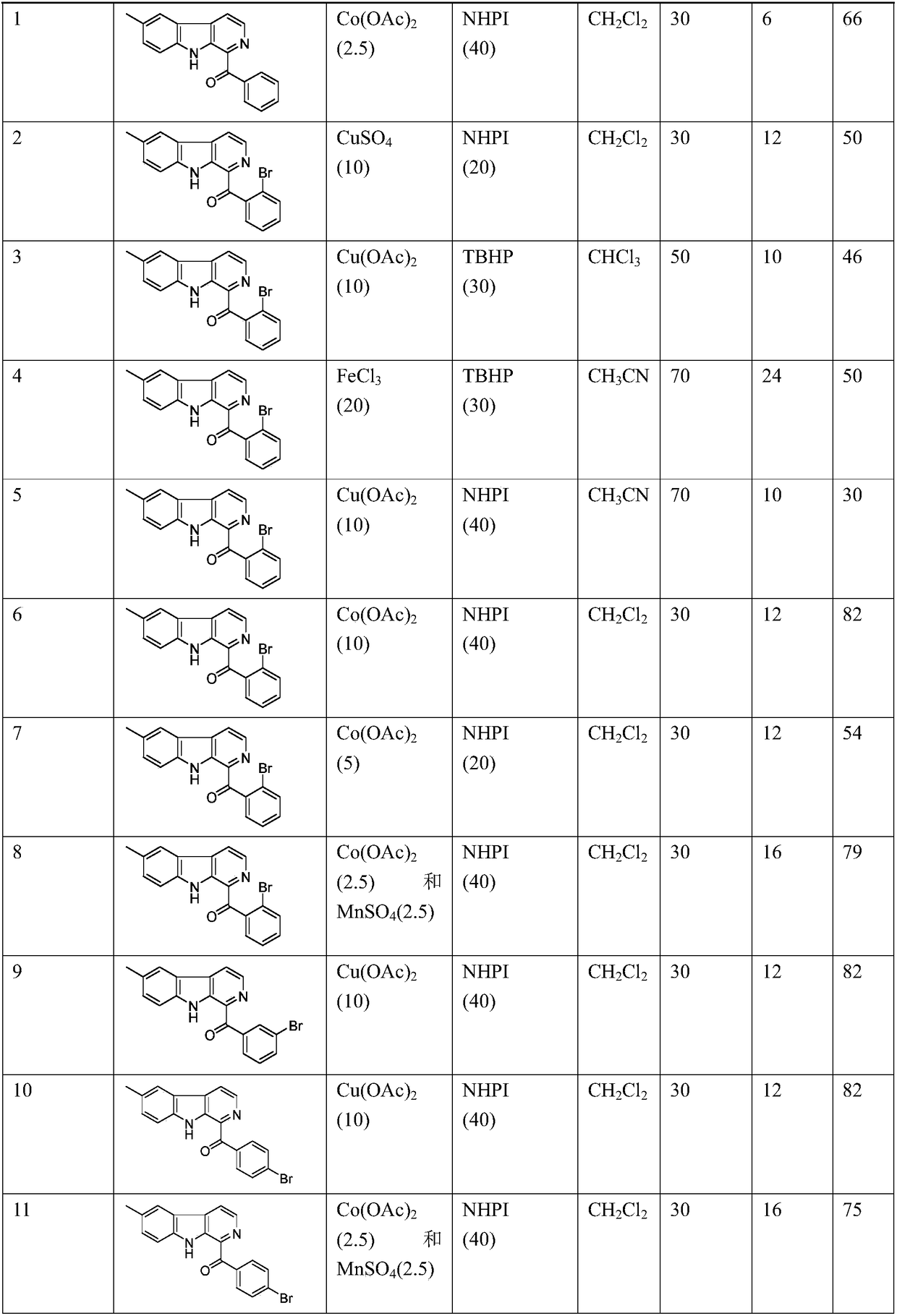
[0021] The preparation method of the examples in the present invention is as follows: in an organic solvent, add methyl-β-carboline, catalyst and condensation agent, react at a certain temperature for a certain period of time, and obtain a crude product after filtering the reaction mixture, and the crude product is re- The product is obtained after crystallization or column purification. Embodiment 1-15, methyl-beta-carboline reaction material used, catalyst, initiator, organic solvent, reaction temperature, reaction time and productive rate are shown in Table 1. The compound structures of the products are shown in Table...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com