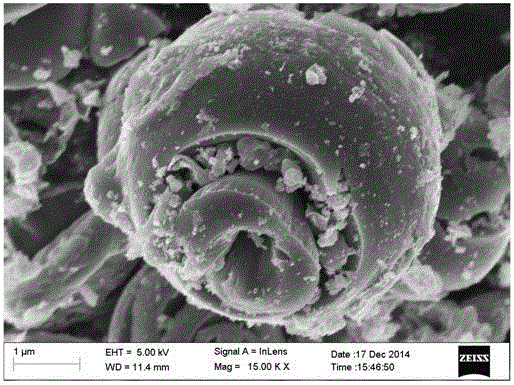
Preparation method of N/S-codoped carbon microspheres with high oxygen reduction catalytic activity
A catalytically active, co-doping technology, applied in electrical components, battery electrodes, circuits, etc., can solve the problems of no oxygen reduction catalytic electrochemical performance, no electrochemical performance, unfavorable large-scale synthesis, etc. The effect of strong oxygen reduction catalytic ability, strong tolerance and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] In a nitrogen-protected glove box, add 0.2 g of cetyltrimethylammonium chloride, 0.5 g of analytically pure sublimed sulfur and 4 mL of ethylenediamine to 6 mL of toluene in sequence, stir for 2 minutes, and put the mixture into a volumetric Put it in a 15mL stainless steel reaction kettle, seal it; then place the reaction kettle in a crucible boiler, heat it at 300°C for 6h, then wait for the reaction kettle to cool down to room temperature naturally, and take out the mixture. The above mixture was washed 4 times successively with absolute ethanol, dilute hydrochloric acid and distilled water, filtered, and then vacuum-dried at 60°C for 12 hours, and the obtained product and analytically pure potassium hydroxide solid were mixed uniformly at a weight percentage of 1:2, and then Put it in a box-type atmosphere furnace and activate it in an argon atmosphere at 500°C for 2 hours, wash the activated product with dilute hydrochloric acid and distilled water for 5 times, and ...
Embodiment 2
[0029] In a nitrogen-protected glove box, 1 g of cetyltrimethylammonium bromide, 0.5 g of analytically pure TMTD, and 4 mL of acetonitrile were sequentially added to 24 mL of toluene, stirred for 4 minutes, and the mixture was put into a 45 mL volume of stainless steel In the reaction kettle, seal it; then place the reaction kettle in a crucible boiler, heat at 400°C for 18h, then wait for the reaction kettle to cool down to room temperature naturally, and take out the mixture. The above mixture was washed 5 times successively with absolute ethanol, dilute hydrochloric acid and distilled water, filtered, and then vacuum-dried at 80°C for 10 h, and the obtained product and analytically pure potassium hydroxide solid were mixed uniformly in a weight percentage of 1:1, and then placed Activate in a box-type atmosphere furnace at 600°C in an argon atmosphere for 1 hour, wash the activated product with dilute hydrochloric acid and distilled water for 5 times, and then dry it in vacu...
Embodiment 3
[0032]In a nitrogen-protected glove box, 1.5 g of cetyltrimethylammonium bromide, 2 g of analytically pure thiourea and 4 mL of acetonitrile were sequentially added to 24 mL of xylene, stirred for 6 minutes, and the mixture was placed in a volume of 45 mL In a stainless steel reaction kettle, seal it; then place the reaction kettle in a crucible boiler, heat it at 500°C for 24h, then wait for the reaction kettle to cool down to room temperature naturally, and take out the mixture. The above mixture was washed 6 times successively with absolute ethanol, dilute hydrochloric acid and distilled water, filtered, and then vacuum-dried at 100°C for 6 h, and the obtained product and analytically pure potassium hydroxide solid were mixed uniformly at a weight percentage of 2:1, and then placed Activated in a box-type atmosphere furnace in an argon atmosphere at 800°C for 0.5h, washed the activated product with dilute hydrochloric acid and distilled water for 4 times, and then dried in v...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com