Preparation method of rare earth metal organic framework fluorescent probe and application of rare earth metal organic framework fluorescent probe in detection of trivalent arsenic
A fluorescent probe and organic framework technology, applied in the field of fluorescence sensing, to achieve the effect of ultra-sensitive and selective fluorescence detection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
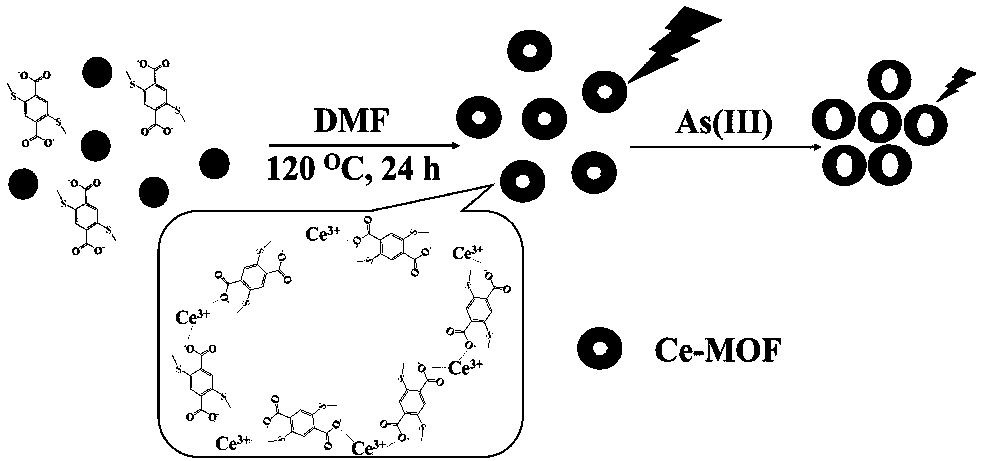
[0018] Preparation of Rare Earth Metal Organic Framework Fluorescent Probes
[0019] (1) Preparation of 2,5-dimethylmercaptoterephthalic acid: add 2.99g potassium permanganate, 1.0g 2,5-dibromo-p-xylene and 1.5g diatomaceous earth to 50mL water and 50mL tert-butanol In the mixed solution, stir mechanically at 100°C for 36 hours, after cooling to 70°C, slowly add ethanol dropwise until the solution turns from red to brown, collect the filtrate by filtration, concentrate to 20mL at 60°C, add 10mL concentrated hydrochloric acid to acidify, filter , washed with ethanol, the white solid was collected and dried in vacuo; 0.25 g of the dried white solid was dissolved in N,N-dimethylformamide (DMF), 0.14 g of sodium methyl mercaptide was added, and stirred at room temperature for 10 minutes. Then extract with chloroform, wash with water, dry over anhydrous magnesium sulfate, and finally dry under vacuum at 60°C to obtain a yellow powder, which is made into 2,5-dimethylmercaptoterephth...
Embodiment 2
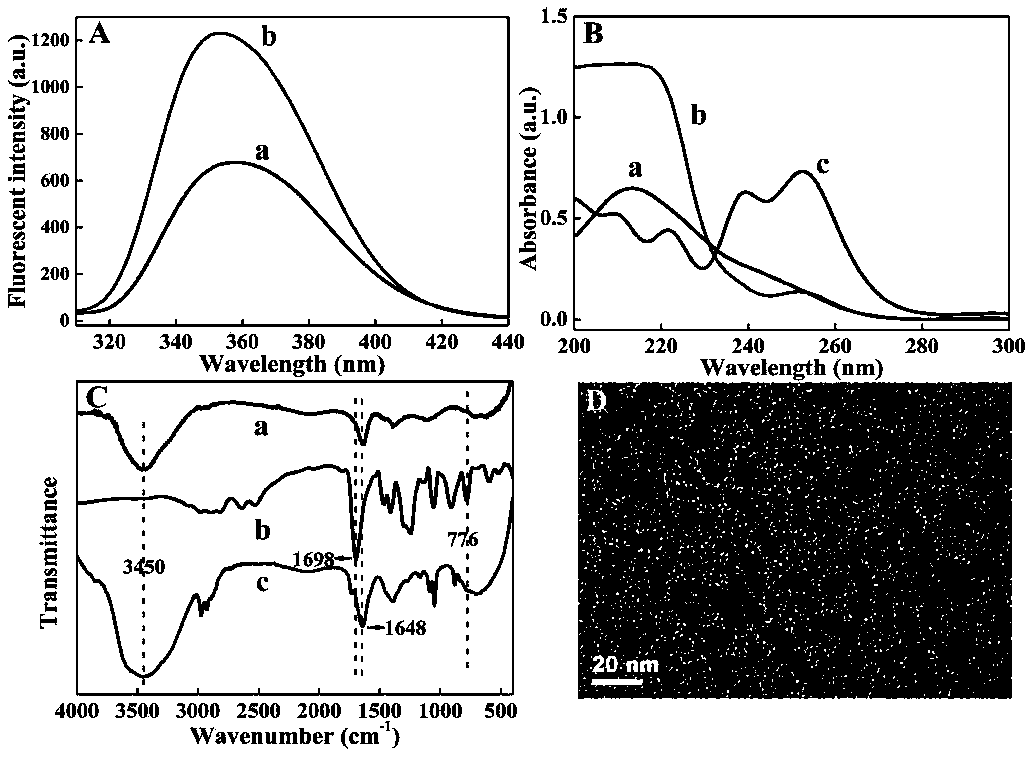
[0024] Interaction mechanism between Ce-MOFs and As(III)
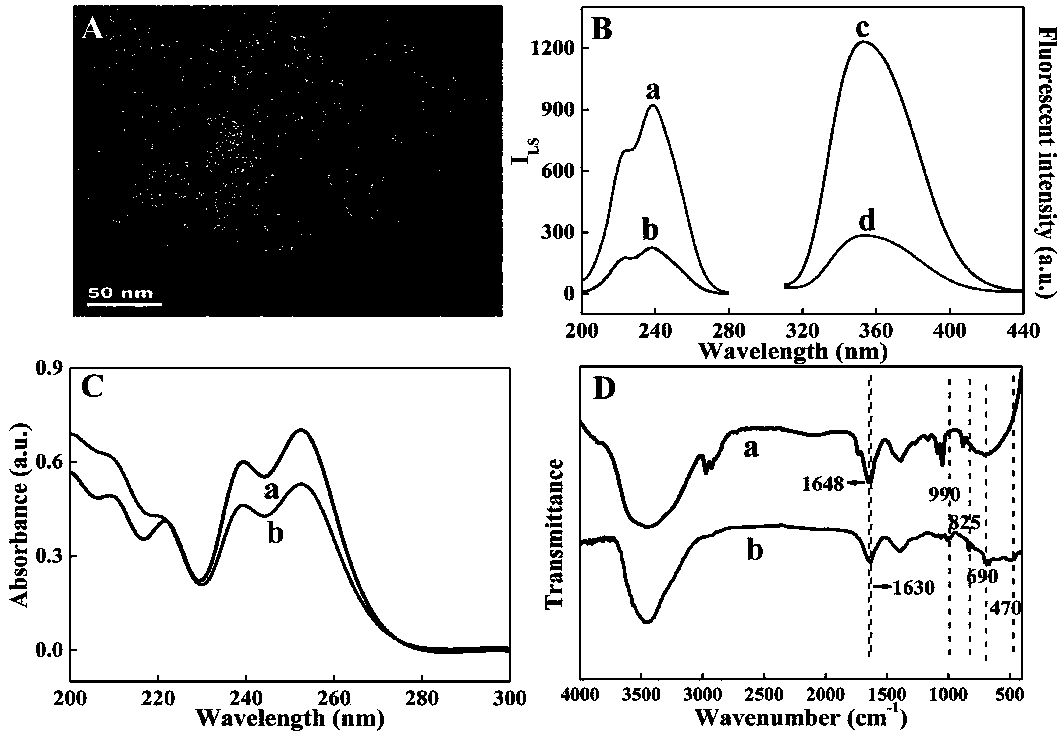
[0025] The interaction mechanism between Ce-MOFs and As(III) was characterized by means of TEM, scattering spectroscopy, fluorescence spectroscopy, UV-visible absorption spectroscopy and infrared spectroscopy. Depend on image 3 A shows that when As(III) is added to Ce-MOFs, the Ce-MOFs nanoparticles originally dispersed in the solution aggregate, which may be due to the interaction between Ce-MOFs and As(III) that brings Ce-MOFs closer. Distance between MOFs. The interaction between Ce-MOFs and As(III) was characterized by dynamic light scattering spectroscopy and fluorescence spectroscopy ( image 3 B), the dynamic light scattering intensity of Ce-MOFs is small (curve b), and when As(III) is added to Ce-MOFs, the dynamic light scattering intensity is enhanced (curve a), indicating that Ce-MOFs and As(III) Coordination aggregation occurs and the particle size increases. In addition, Ce-MOFs have a strong emission ...
Embodiment 3
[0028] Application of Ce-MOFs to As(III) Detection
[0029] Add 20 μL of As(III) solutions of different concentrations to 10 μL of 4 mg / mL rare earth metal organic framework fluorescent probe solution, dilute the total volume of the solution to 200 μL with ultrapure water, incubate at room temperature for 40 minutes, and measure the solution when the excitation wavelength is 280 nm. of fluorescence. Depend on Figure 4 It can be seen that as the concentration of As(III) increases, the fluorescence intensity of Ce-MOFs decreases gradually, and the concentration of As(III) is related to the fluorescence intensity of the fluorescent probe (1-F / F 0 , F is the fluorescence intensity of Ce-MOFs in the presence of As(III), F 0 is the fluorescence intensity of Ce-MOFs) shows good linearity in the range of 1ppb-80ppb, and the detection limit is 0.65ppb, which can be used for ultrasensitive detection of As(III) in water samples.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com