Hybridoma cell strain and glycocholic acid monoclonal antibody and detection kit based on hybridoma cell strain
A hybridoma cell line and monoclonal antibody technology, applied in the field of detection, can solve the problem of low titer, achieve high sensitivity, fast response, and convenient sample operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Example 1: Preparation of Monoclonal Antibody
[0042] (1) Animal immunity
[0043] The immunogen is pure glycocholic acid, which is directly dissolved in PBS to make a concentration of 1 mg / mL. Take 0.5mL of the above solution and emulsify it with an equal volume of Freund's complete adjuvant, inject it into Balb / C mice (5 6-week-old Balb / C female mice, 18-22g.), 0.5mL / mouse, take subcutaneous Multiple injections and intraperitoneal injections. The immune antigen was emulsified with complete Freund's adjuvant for the first immunization, and then emulsified with incomplete Freund's incomplete adjuvant, with an interval of 15 days between each immunization. Booster immunization was carried out on the 15th and 29th day, and the immunization dose was the same as the initial immunization. Three days before fusion, a final pulse immunization was performed with antigen without adjuvant. After the third immunization, blood was collected from the tail vein of the mice, and ...
Embodiment 2
[0050] Example 2: Screening of hybridoma cells and their subcloning
[0051] After fusion, change 100 μL of the HAT medium half solution every three days, and do not pipette. After 10 days, replace all the HAT medium with HT medium, and change the whole HT medium every 3 days. After culturing for about 20 days, replace it with a general complete medium.
[0052] Observe the cell growth every day. On the 10th day, the cells cover 1 / 2 of the culture well. Take the supernatant for indirect ELISA assay to detect whether antibodies are produced.
[0053] Prepare hybridoma cell suspension: Gently blow off the hybridoma cells in the positive wells detected by ELISA, pipette the hybridoma cells into a 10mL centrifuge tube, count, calculate, and dilute the cell concentration with complete medium to 200 cells / mL, take out 2 mL and add it to 2 mL of complete medium containing feeder cells, and the concentration of hybridoma cells becomes 100 cells / mL;
[0054] Limiting dilution: 100 μ...
Embodiment 3
[0056] Example 3: Ascites induction and antibody purification
[0057] Induction of ascites: 5 Balb / C mice were taken, and each mouse was intraperitoneally injected with 0.5 mL of Freund's incomplete adjuvant about ten days before hybridoma cell inoculation. At the same time, culture the monoclonal antibody-secreting cell line in a cell culture flask to the logarithmic phase, centrifuge at 1000rpm for 5min, take the precipitate and resuspend it with 1640 basal medium, and adjust the number of cells to 1×10 6 cells / mL, inject 0.5mL hybridoma cells intraperitoneally into the above mouse, and wait for the mouse abdomen to be obviously enlarged and move slowly, about 10-13 days later. Disinfect the abdominal skin with alcohol cotton balls, extract the ascites with a 5mL syringe, centrifuge the ascites at 4°C and 1000rpm for 10min, take the supernatant, aliquot it and store it in a -20°C refrigerator for later use.
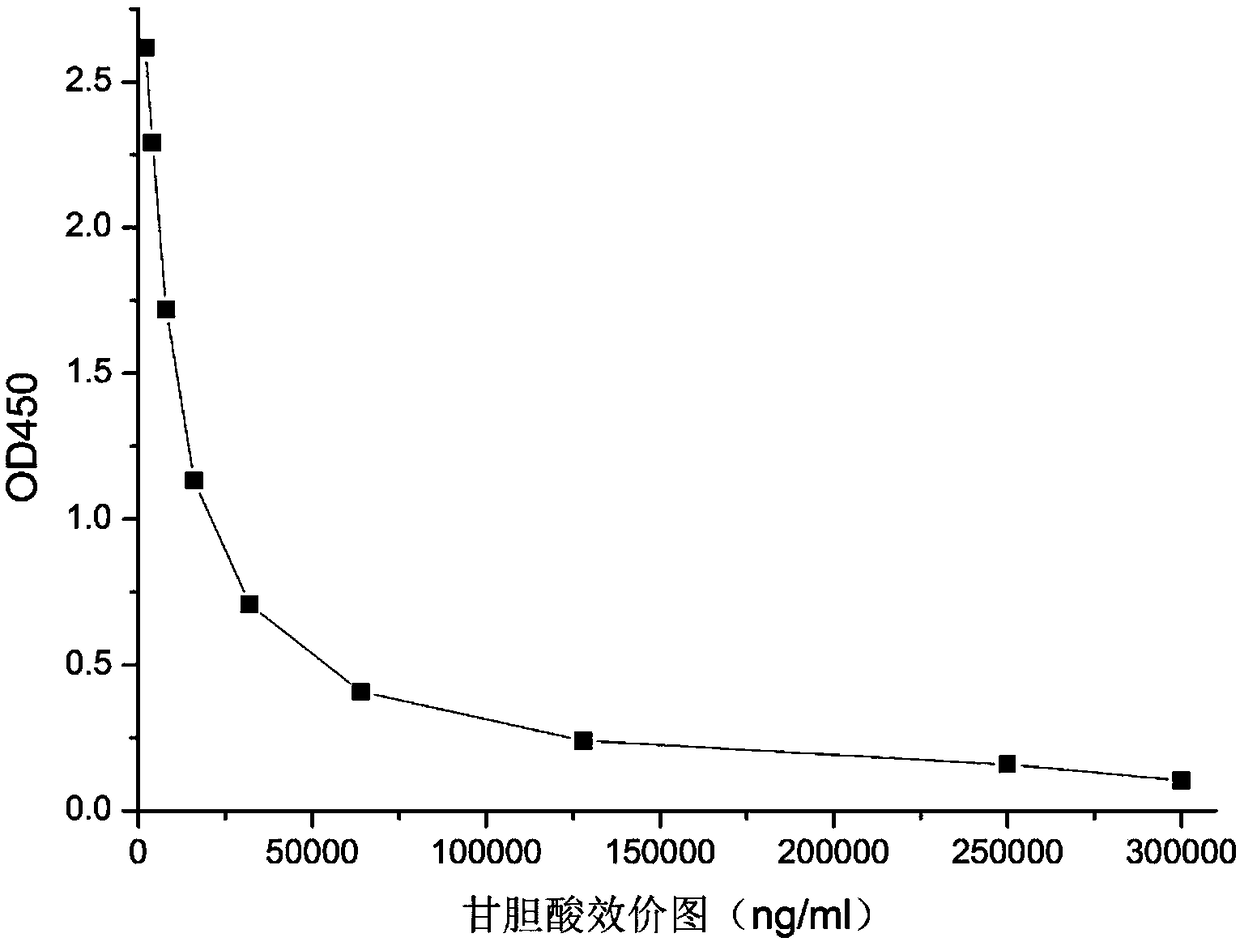
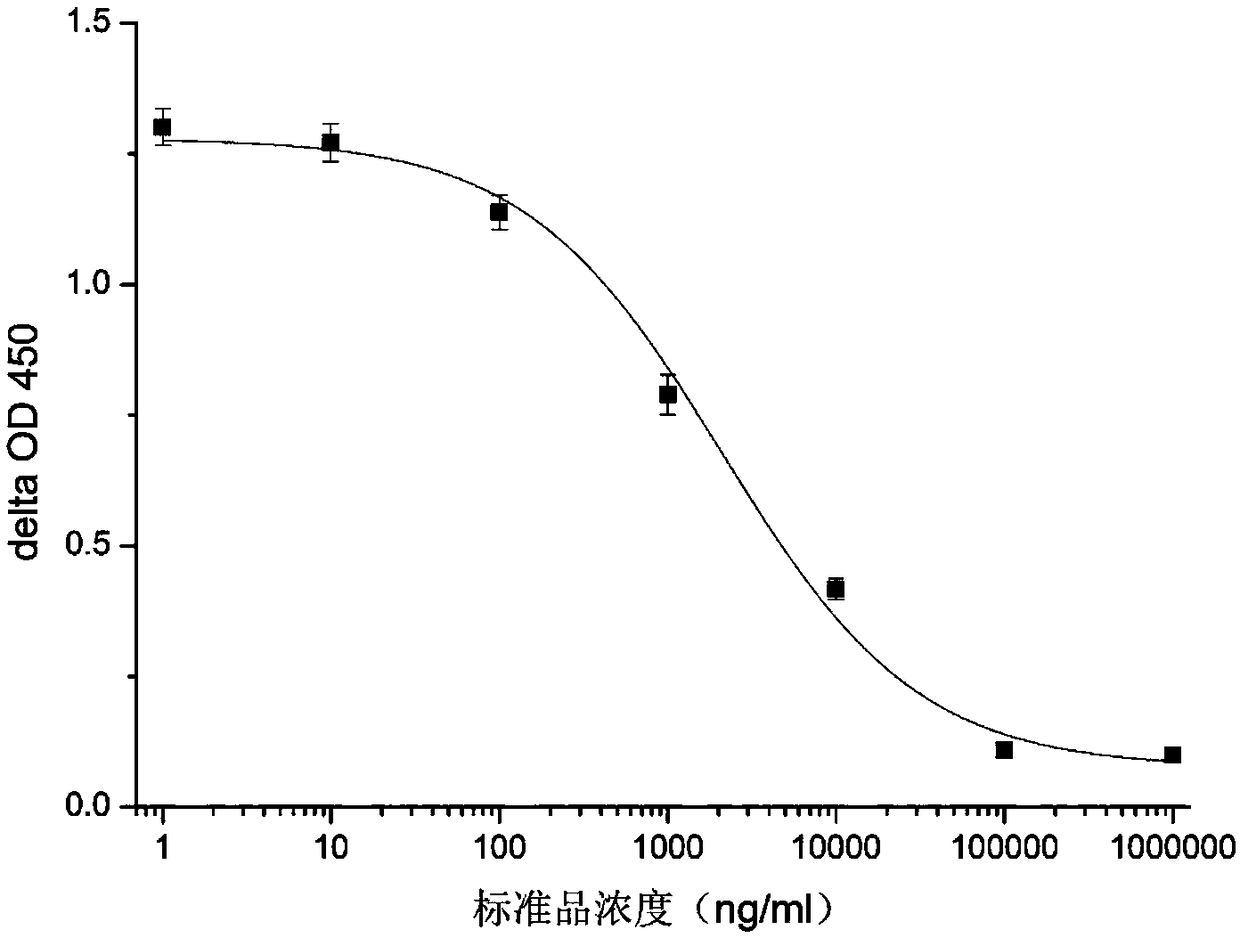
[0058] Purification of Glycocholic Acid Antibodies: Antibodies f...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com