Marker assisting epilepsy diagnosis and detection kit of marker
A kit and marker technology, which can be used in biological testing, disease diagnosis, microbial determination/examination, etc., and can solve the problem of lack of epilepsy diagnostic markers.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0063] 1 Detection of FASN mutation in a family with epilepsy
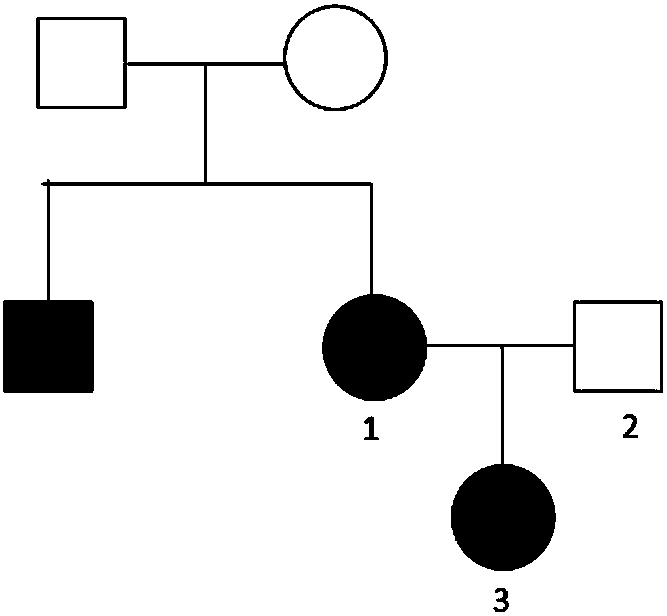
[0064] 1.1 Subjects: The proband and family members of Huang's epilepsy family from Jiangjin District, Chongqing City. The genetic map of the family is as follows: figure 1 As shown, the test objects include 2 epilepsy patients (member 1 and 3 in the figure), and 1 normal family member (member 2 in the figure); No. 1 and No. 3: generalized tonic-clonic seizures; No. 2: no epilepsy sick. A detailed physical examination was performed on all family members No. 1-3, and blood samples were collected from each person after signing the informed consent.
[0065] 1.2 Sequencing:
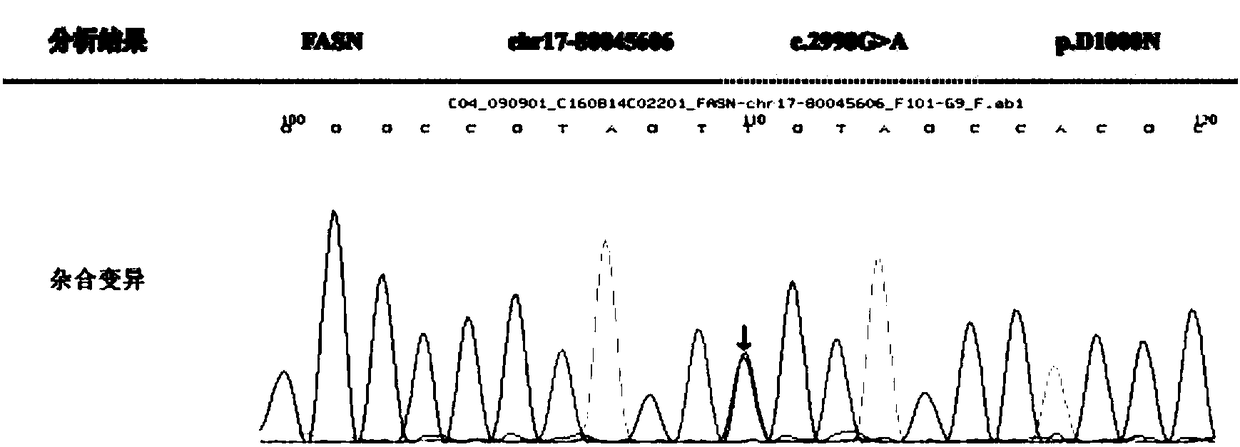
[0066] The collected blood samples were sent to Beijing Mikino Gene Technology Co., Ltd. for sequencing for epilepsy gene mutation screening and verification, and the relationship between clinical phenotype and genotype was analyzed. The results are as follows:
[0067] The measured sequence was compared with the normal FASN standard sequence...
Embodiment 2
[0073] This embodiment provides a kit for detecting the FASN 2998th site mutation, which includes: a primer for detecting whether the FASN2998 site has a mutation G2998A, a forward primer: AAGCTGCATGCCTAGCTGTG, a reverse primer: GAACGGCAACCTGGTAGTGAG; and a PCR amplification reagent: 10×Buffer (containing 15mM Mg 2+ ), dNTP (2.5Mm), high-fidelity DNA polymerase pfu DNA polymerase (5U / μl) and ddH 2 O.
[0074] The method for detecting whether the mutation G>A occurs at the 2998th position of FASN by using the kit mainly includes the following steps:
[0075] (1) Extract sample DNA and use it as a template to perform PCR reaction using the above-mentioned PCR reaction kit.
[0076] (2) Detection of multiple PCR products: electrophoresis was performed on the PCR amplification products with agarose gel to detect whether the PCR amplification was successful, and the length of the amplification was 385 bp.
[0077] (3) The PCR product is directly sequenced, and the sequence resul...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com