ZIKV (zika virus) vaccine and preparation method thereof
A Zika virus and vaccine technology, applied in the field of vaccines, to achieve the effect of improving infectivity, good virus infection and transmission, and strong immunogenicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Example 1 Zika virus vaccine and its preparation method
[0044] In an embodiment of the Zika virus vaccine of the present invention, the Zika virus vaccine is a gene vector capable of expressing Zika virus membrane protein prM / M, envelope protein E and non-structural protein NS1, wherein the vector is a shuttle Plasmid pGA1; the vector contains the coding sequence of Japanese encephalitis virus signal peptide, the coding sequence of membrane protein prM / M, the coding sequence of envelope protein E, the 2A self-splicing sequence and the recombination of the coding sequence of non-structural protein NS1 in sequence The recombinant sequence is inserted into the open reading frame of the non-structural protein NS1, and the coding sequences of the membrane protein prM / M, the envelope protein E and the non-structural protein NS1 are optimized according to the mammalian codon preference.
[0045] The method for preparing the Zika virus vaccine includes the following steps:
[0046]...
Embodiment 2
[0048] Example 2 Construction of a recombinant adenovirus vector carrying the coding sequence of the Zika virus main antigen
[0049] 1. Recombinant adenovirus vector plasmid construction.
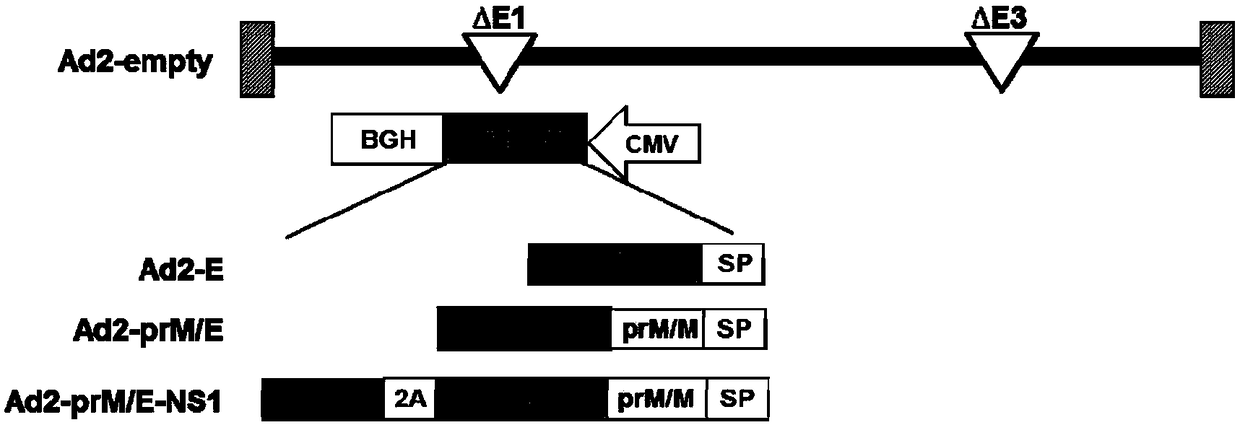
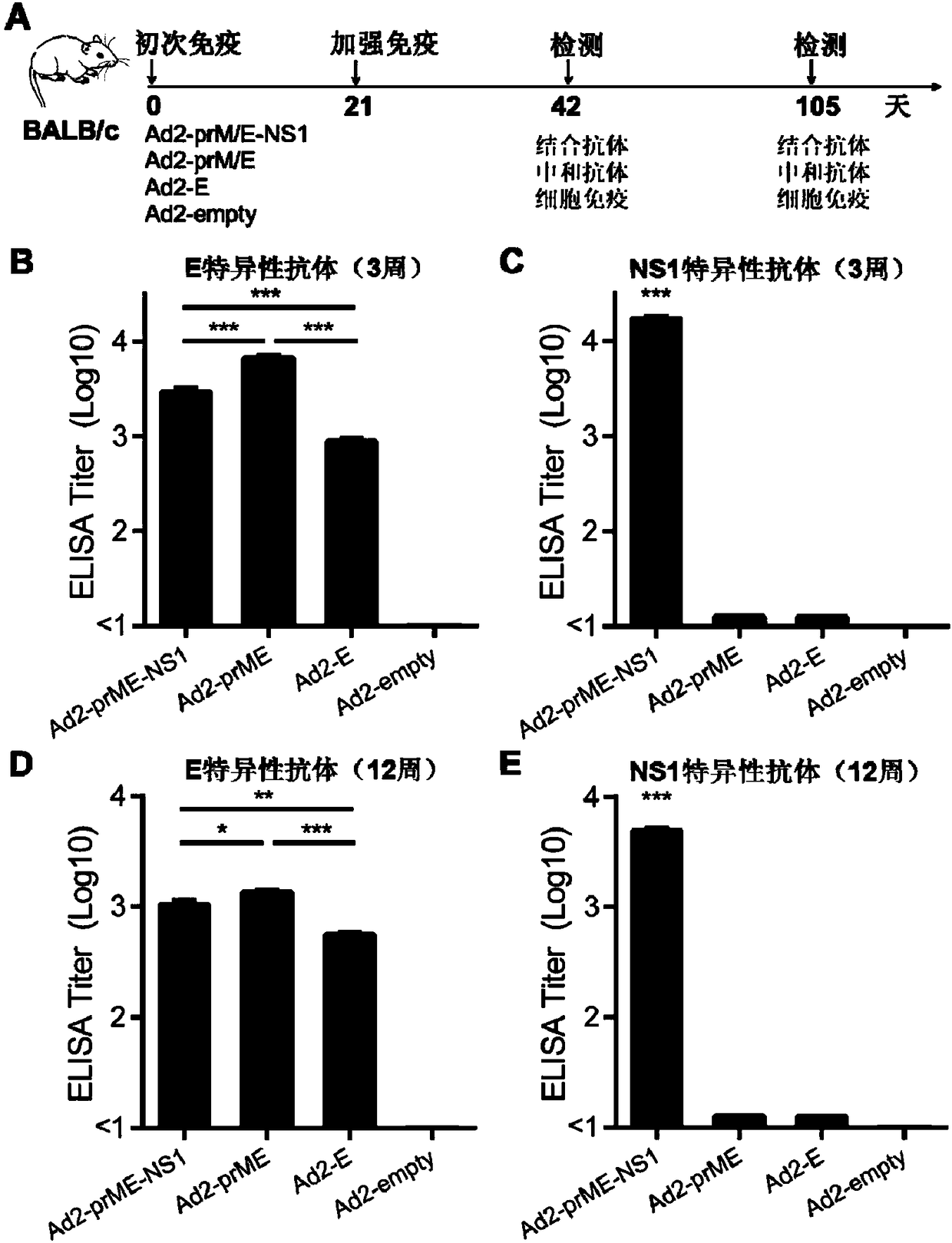
[0050] Zika virus E protein, prM / M protein, and NS1 protein sequences are from Zika virus isolate 1_0080_PF (GenBank No: ANO46313.1). The coding gene sequences of these proteins were optimized based on mammalian cell codons and obtained through total gene synthesis (GenScript, China). The fusion gene sequence Ad2-prM / E-NS1 consists of (5' end to 3'end): JEV (Japanese Encephalitis Virus) signal peptide, prM / M, E, 2A self-splicing sequence, NS1; Ad2-prM / The map of E-NS1 sequence is as figure 1 As shown, the base sequence of the fused prM / E-NS1 is shown in SEQ ID NO. 1, and the corresponding amino acid sequence is shown in SEQ ID NO.2.
[0051] In order to compare the beneficial effects of the recombinant Zika virus containing the NS1 antigen, this example also constructed a recombinant adenoviru...
Embodiment 3
[0054] Example 3 Identification of antigen expression of recombinant adenovirus vector Zika virus vaccine
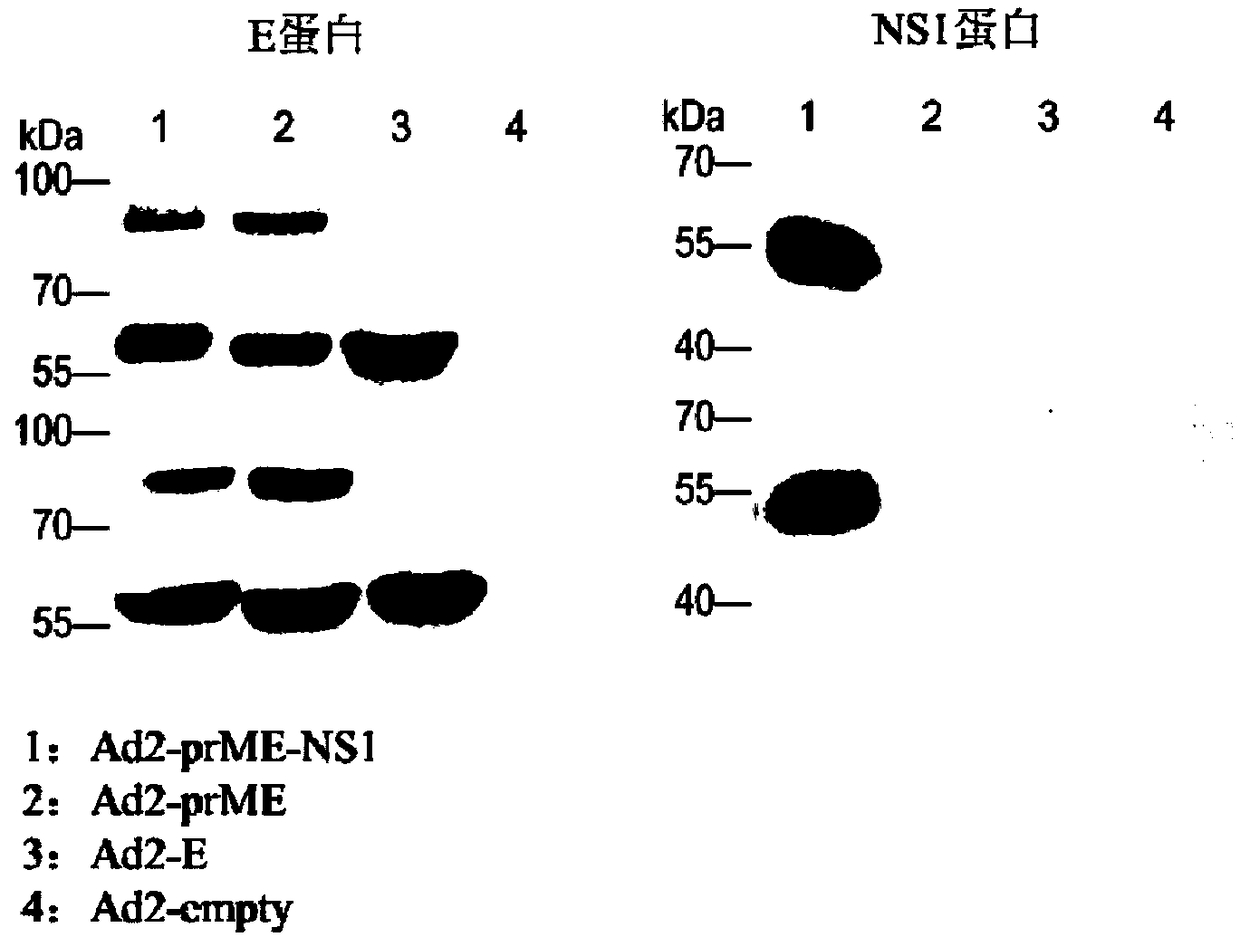
[0055] Vero cells were infected with Ad2-E, Ad2-prM / E, Ad2-prM / E-NS1 and Ad2-empty empty vector controls respectively, and the infection dose was 100 viral particles (vp) per cell. 48 hours after infection , Collect infected cells and culture supernatant. Western-blot was used to detect the expression of antigen protein. The protein samples were electrophoresed by SDS-PAGE gel, transferred to PVDF membrane, blocked with PBST containing 5% skimmed milk powder for 1 hour at room temperature, and then incubated with anti-Zika virus E protein antibody or anti-Zika virus NS1 protein antibody at room temperature for 1 hour . Then, the goat anti-human secondary antibody labeled with horseradish peroxidase was incubated. Finally, the horseradish peroxidase substrate was used for color development.
[0056] Experimental results: such as figure 2 As shown, Ad2-E, Ad2-prM / E, Ad2-pr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com