Dendritic organic boron crosslinker as well as preparation method and application thereof
A dendritic and organoboron technology, applied in the direction of organic chemistry, chemical instruments and methods, drilling compositions, etc., can solve the problem of short delay time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0022] The invention provides a kind of preparation method of dendritic organic boron crosslinking agent, comprises the following steps:
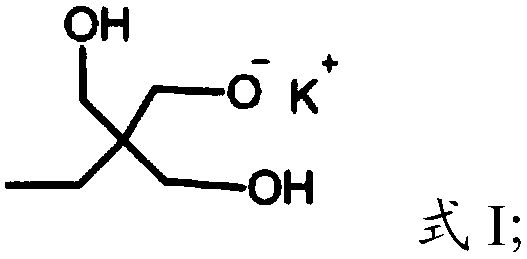
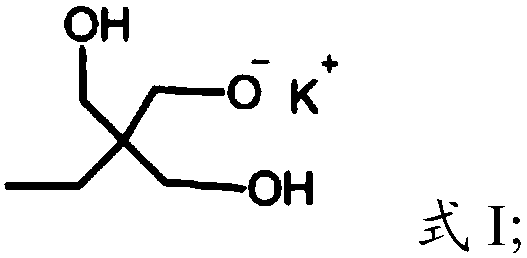
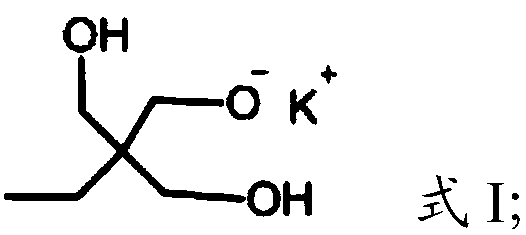
[0023] (1) Under the protection of argon, the compound having the structure shown in formula I is polymerized with glycidol to obtain dendritic polyhydroxy macromolecules,
[0024]
[0025] (2) Under the condition that the pH value is 11-13, the dendritic polyhydroxy macromolecule obtained in the step (1) is reacted with a boron-containing compound to obtain a dendritic organoboron crosslinking agent.
[0026] In the present invention, under the protection of argon, the compound having the structure shown in formula I is polymerized with glycidol to obtain dendritic polyhydroxy macromolecules. The present invention has no special limitation on the source of the compound shown in formula I, and it can be prepared by using commercially available products or conventional preparation methods well known to those skilled in the art, such as re...
Embodiment 1
[0042] In a three-necked flask, 0.67 mL of a methanol solution of potassium methoxide (mass concentration 25%) and 1.2 g of trimethylolpropane were mixed and stirred evenly, and the methanol was distilled off under reduced pressure. Install mechanical stirring, place the three-necked bottle in an oil bath, fill it with high-purity argon, and heat it to 95°C. Add organic solvent dioxane 120mL. Then add 120 mL of glycidol monomer, after 12 hours of reaction, add excess methanol to dilute. The diluted mixed liquor is treated with cation exchange resin. The product is then precipitated out using excess acetone to yield dendritic polyhydroxy macromolecules.
[0043]Mix 5 g of dendritic polyhydroxy macromolecules with 5 g of water to obtain an aqueous solution of dendritic polyhydroxy macromolecules, then mix 15 g of boric acid with 100 g of water to obtain an aqueous solution of boric acid, mix the aqueous solution of dendritic polyhydroxy macromolecules with an aqueous solution ...
Embodiment 2
[0047] The steps for preparing dendritic polyhydroxy macromolecules are the same as in Example 1.
[0048] Mix 5 g of dendritic polyhydroxy macromolecules with 5 g of water to obtain an aqueous solution of dendritic polyhydroxy macromolecules, then mix 5 g of boric acid with 100 g of water to obtain an aqueous solution of boric acid, mix the aqueous solution of dendritic polyhydroxy macromolecules with an aqueous solution of boric acid, and use hydrogen Sodium oxide solution adjusts the pH value of the system to 13, heats to 60° C. for reaction for 1 h, distills and concentrates the reaction product under reduced pressure, adds salt to precipitate a solid product, separates the precipitate, and dries to obtain a dendritic organoboron crosslinking agent.
[0049] Prepare 100 mL of 0.45% guar gum base solution, let it stand for 2 hours, and adjust the pH to 11.0; add 0.1 mL (mass concentration: 3.5%) of the above-mentioned dendritic organoboron cross-linking agent solution, and d...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com