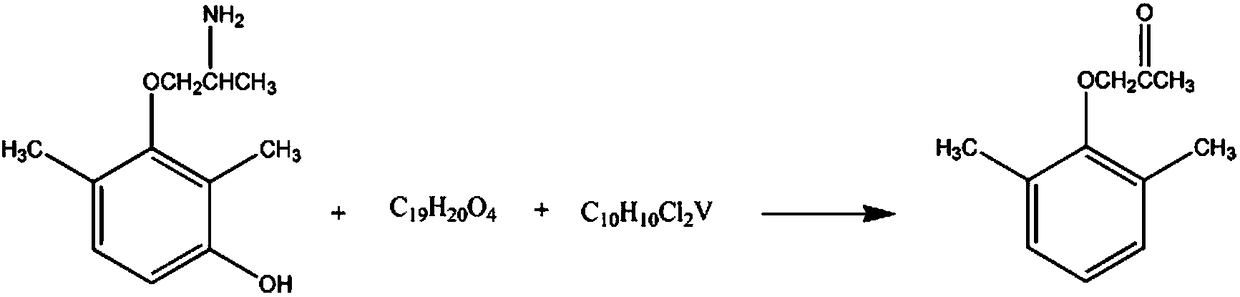Synthetic method for antiarrhythmic drug intermediate 1-(2,6-dimethoxy)-2-propanone
An antiarrhythmic and dimethoxy technology, which is applied in the synthesis of antiarrhythmic drug intermediate 1--2-acetone and the preparation of pharmaceutical intermediates, can solve the problems of high risk factor, large environmental pollution, complex process, etc. problem, to achieve the effect of increasing reaction yield, shortening reaction time and improving reaction yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] The synthetic method of antiarrhythmic drug intermediate 1-(2,6-dimethoxy)-2-propanone comprises the steps:
[0017] A, add 2mol of 1-(2,6-dimethyl-3-hydroxyl-phenoxy)-2-aminopropane in the reaction vessel, 2L mass fraction is 15% potassium nitrate solution, control stirring speed 250rpm, Raise the temperature of the solution to 35°C, react for 60 minutes, add 3 mol of butyl benzyl phthalate solution with a mass fraction of 40%, and continue the reaction for 30 minutes;
[0018] B. Add 2 mol of dicyanoyl vanadium dichloride, 1.3L of 20% sodium sulfate solution by mass fraction, react for 60 minutes, lower the temperature to 10°C, and let stand for 30 minutes. % sulfur hexafluoride solution, washed with 65% 2-chlorophenol solution, recrystallized in 80% methyl chloride solution, and dehydrated with anhydrous magnesium sulfate dehydrating agent to obtain the finished product 1-(2, 6-dimethoxy)-2-propanone 323.96, yield 91%.
Embodiment 2
[0020] The synthetic method of antiarrhythmic drug intermediate 1-(2,6-dimethoxy)-2-propanone comprises the steps:
[0021] A, add 2mol of 1-(2,6-dimethyl-3-hydroxyl-phenoxy group)-2-aminopropane in the reaction vessel, 2L mass fraction is 15% potassium nitrate solution, control stirring speed 260rpm, Raise the temperature of the solution to 37°C, react for 80 minutes, add 3.5 mol of butyl benzyl phthalate solution with a mass fraction of 47%, and continue the reaction for 40 minutes;
[0022] B, add 2.5mol dicyclovanadium dichloride, 1.3L mass fraction is 23% sodium sulfate solution, react for 70min, lower the temperature to 12°C, let stand for 35min, the solution is separated, and the oil layer is separated, and the mass fraction is Washing with 53% sulfur hexafluoride solution, washing with 68% 2-chlorophenol solution in mass fraction, recrystallization in 83% methyl chloride solution, dehydration with anhydrous magnesium sulfate dehydrating agent, to obtain finished produc...
Embodiment 3
[0024] The synthetic method of antiarrhythmic drug intermediate 1-(2,6-dimethoxy)-2-propanone comprises the steps:
[0025] A. Add 2mol of 1-(2,6-dimethyl-3-hydroxy-phenoxy)-2-aminopropane into the reaction vessel, 2L mass fraction is 22% potassium nitrate solution, control the stirring speed to 270rpm, liter Increase the solution temperature to 42°C, react for 90 minutes, add 4 mol mass fraction of 55% butyl benzyl phthalate solution, and continue the reaction for 50 minutes;
[0026] B. Add 3 mol of dicyanoyl vanadium dichloride, 1.3L mass fraction of 26% sodium sulfate solution, react for 80min, lower the temperature to 14°C, let stand for 40min, separate the layers of the solution, separate the oil layer, and use a mass fraction of 56% Washing with sulfur hexafluoride solution, washing with 2-chlorophenol solution with a mass fraction of 72%, recrystallization in a chloromethane solution with a mass fraction of 87%, and dehydrating with anhydrous potassium carbonate dehydr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com