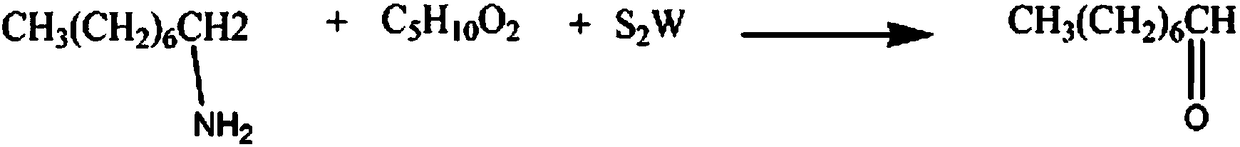Synthetic method for organic synthesis intermediate octanal
A technology of organic synthesis and synthesis method, which is applied in the preparation of organic compounds and the synthesis of octanal, an intermediate of organic synthesis, can solve the problems of non-compliance with green energy saving, economical and environmental protection, complex synthesis process and high heat resistance requirements of equipment, and achieve Avoid the adverse effects of greater environmental pollution, increase the reaction yield, and reduce the heat resistance requirements of the equipment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0012] The synthetic method of organic synthesis intermediate octanal, comprises the steps:
[0013] A: Add 3mol 1-aminooctane and 1.2L anhydrous p-chlorotoluene solution into the reaction vessel, raise the solution temperature to 40°C, control the stirring speed at 170rpm, react for 90min, add 3mol tungsten disulfide powder, and continue the reaction for 2h;
[0014] B: Then add 3 mol of anhydrous isobutyl formate solution, reflux for 80 minutes, lower the temperature to 10°C, let stand for 1 hour, wash with anhydrous 1,3-dichloropropane solution 3 times, and anhydrous diisopropane solution 6 times , recrystallized in anhydrous m-xylene solution, and dehydrated with an activated alumina dehydrating agent to obtain 334.08 g of octanal, a yield of 87%.
Embodiment 2
[0016] The synthetic method of organic synthesis intermediate octanal, comprises the steps:
[0017] A: Add 3mol 1-aminooctane and 1.2L anhydrous p-chlorotoluene solution to the reaction vessel, raise the temperature of the solution to 45°C, control the stirring speed at 180rpm, react for 110min, add 3.6mol tungsten disulfide powder, and continue the reaction 2.8 h;
[0018] B: Then add 5mol anhydrous isobutyl formate solution, reflux for 90min, lower the temperature to 13°C, let it stand for 1.3h, wash with anhydrous 1,3-dichloropropane solution 4 times, and anhydrous diisopropane solution for 7 The second time, recrystallized in anhydrous m-xylene solution, and dehydrated with anhydrous potassium carbonate dehydrating agent to obtain 349.44 g of finished octanal, with a yield of 91%.
Embodiment 3
[0020] The synthetic method of organic synthesis intermediate octanal, comprises the steps:
[0021] A: Add 3mol 1-aminooctane and 1.2L anhydrous p-chlorotoluene solution into the reaction vessel, raise the temperature of the solution to 46°C, control the stirring speed to 210rpm, react for 120min, add 4mol tungsten disulfide powder, and continue the reaction for 3h;
[0022] B: Then add 6mol anhydrous isobutyl formate solution, reflux for 110min, lower the temperature to 15°C, let stand for 2h, wash with anhydrous 1,3-dichloropropane solution for 5 times, and anhydrous diisopropane solution for 8 times , recrystallized in anhydrous m-xylene solution, and dehydrated with an activated alumina dehydrating agent to obtain 360.96 g of octanal, a yield of 94%.
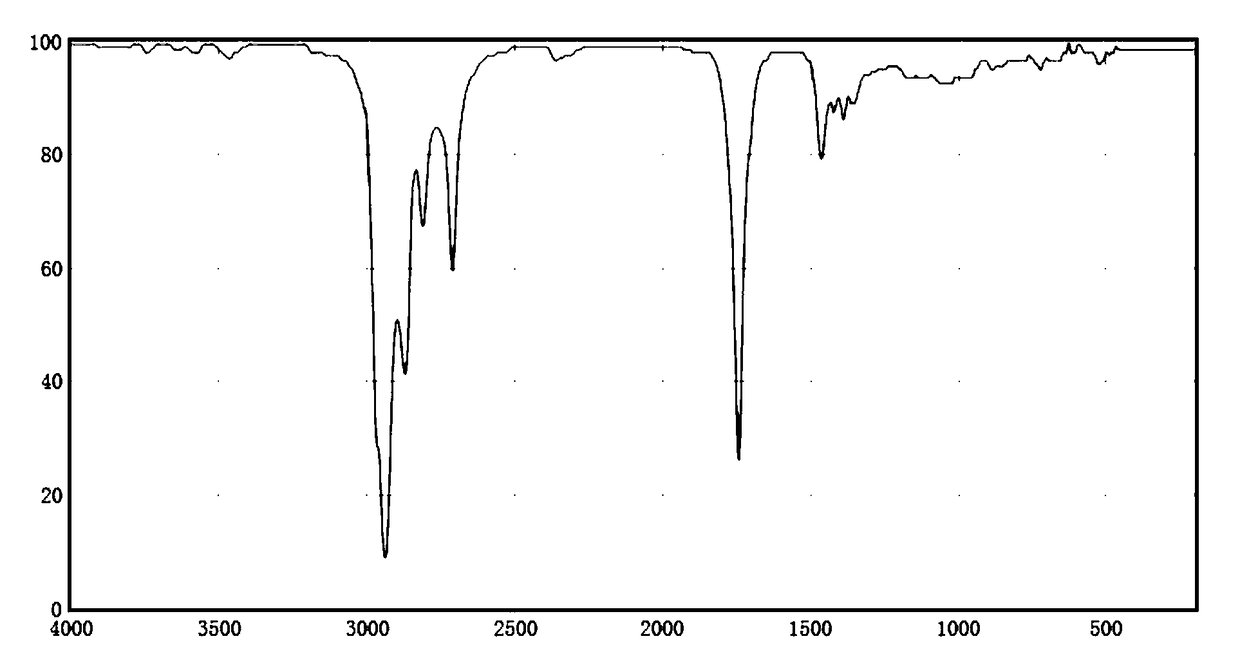
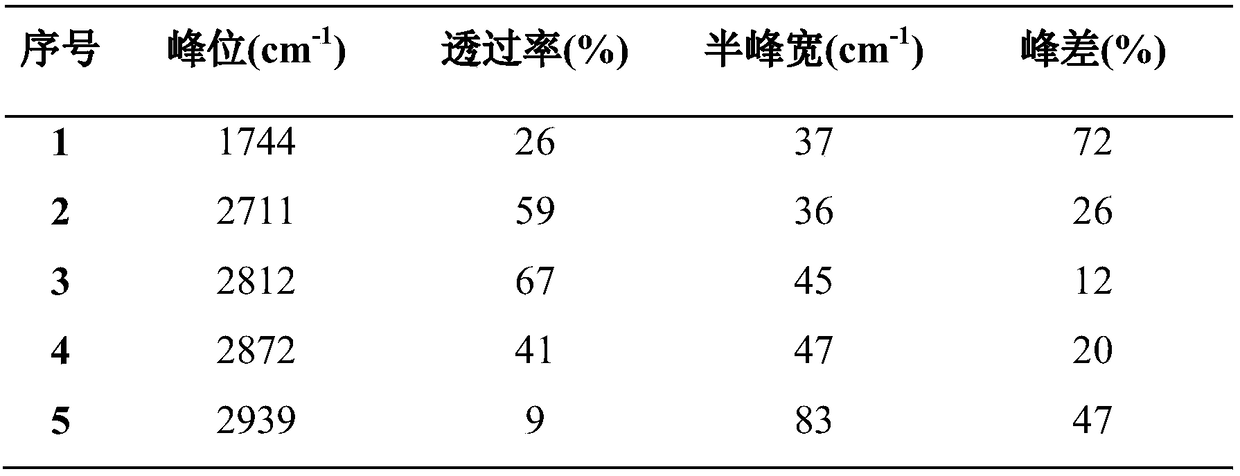
[0023] Table 1 Infrared analysis data
[0024]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com