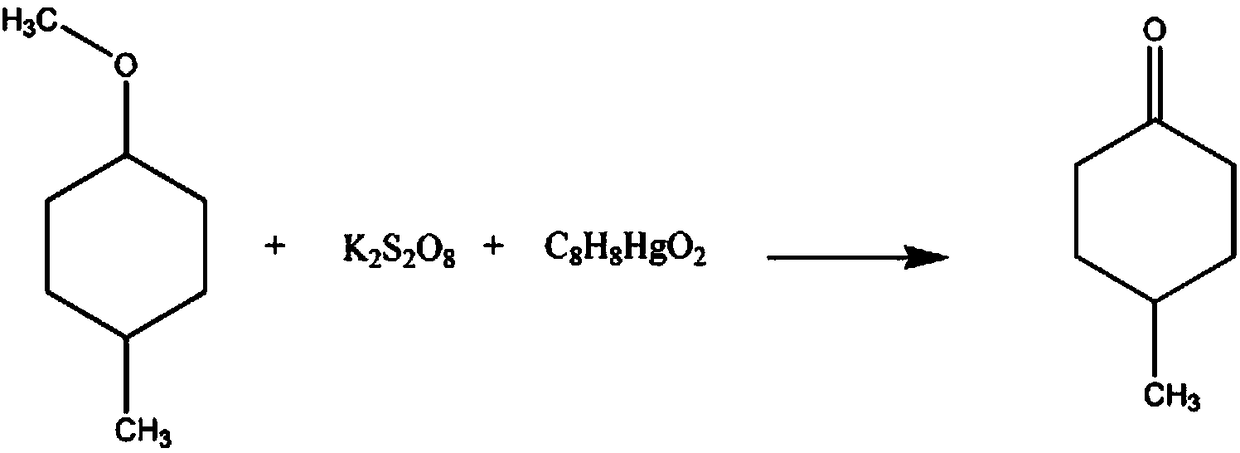Synthetic method for organic synthesis intermediate 4-methylcyclohexanone
A technology for methylcyclohexanone and organic synthesis, applied in the preparation of organic compounds, organic chemistry, chemical instruments and methods, etc., can solve the problem of increased corrosion resistance requirements for reaction equipment, increased risk factors in the synthesis process, and complex synthesis processes and other problems, so as to avoid the increase of corrosion resistance requirements, shorten the reaction time and improve the reaction yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
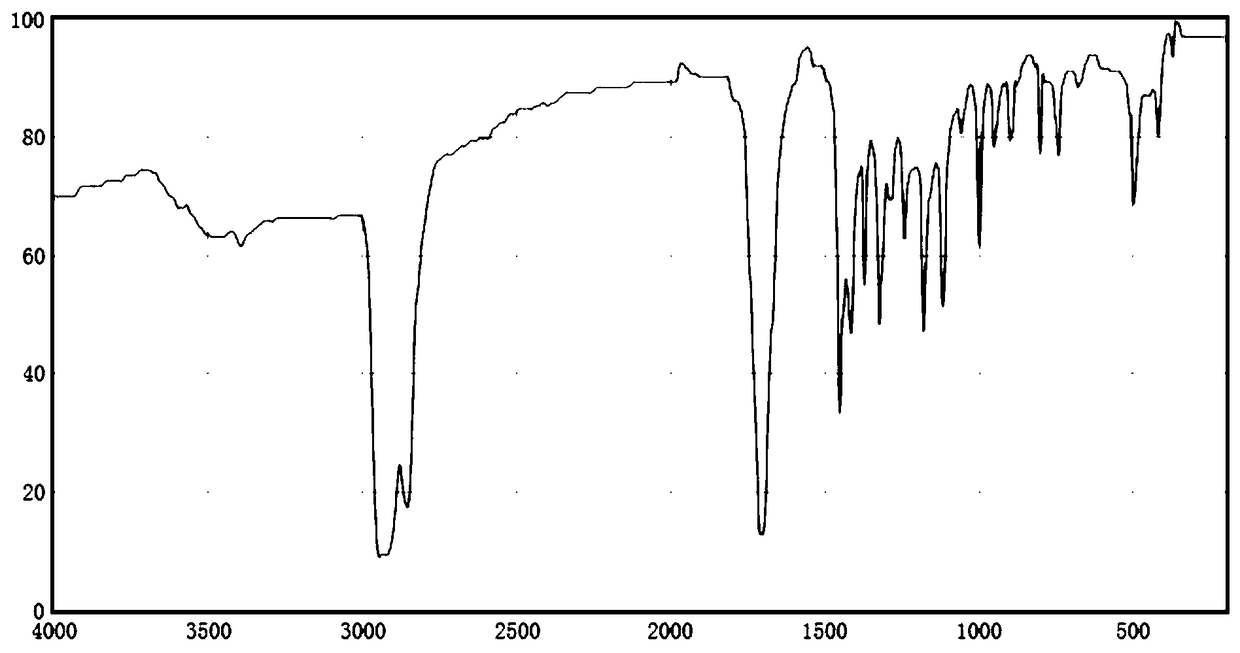
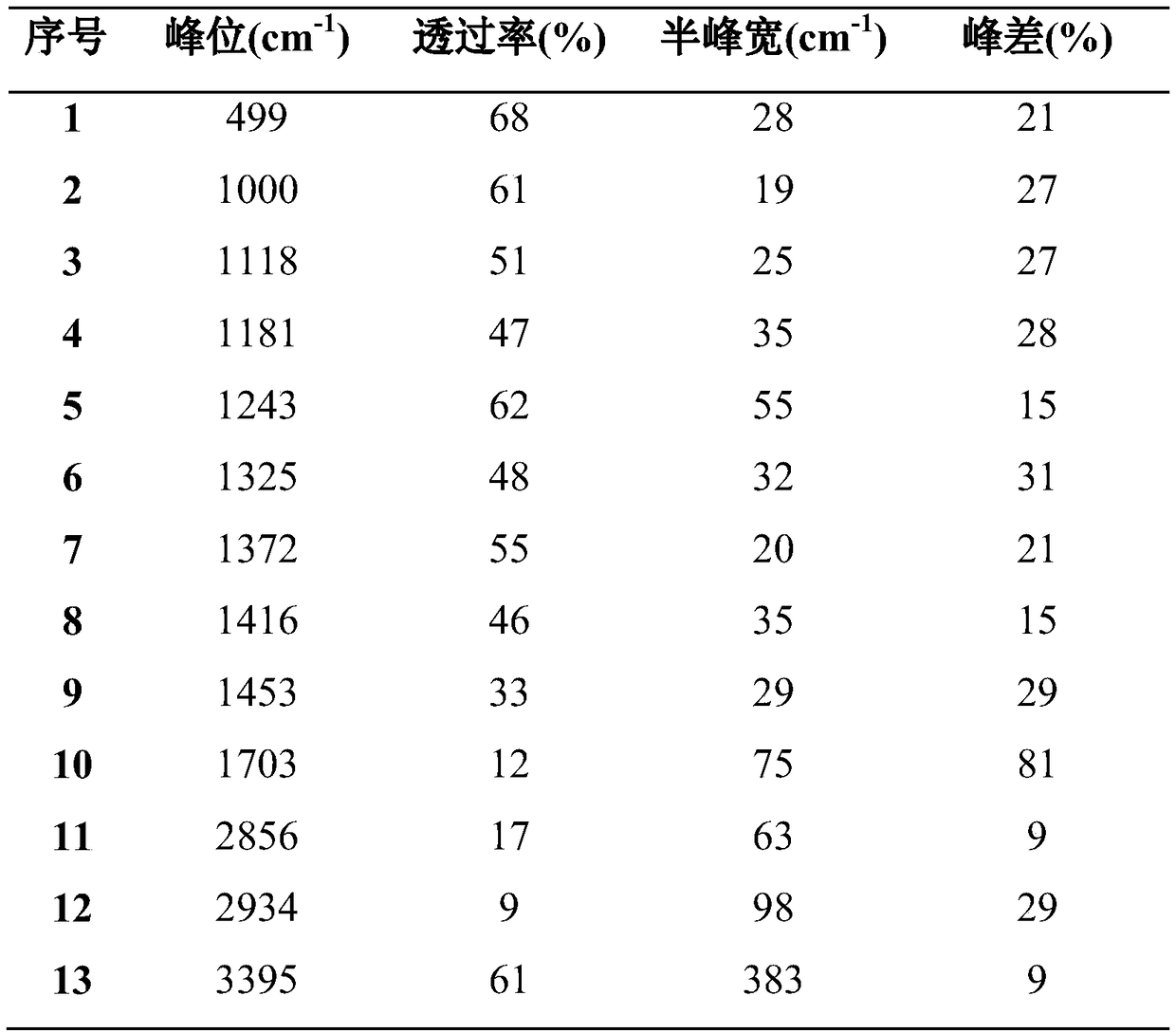
Image
Examples
Embodiment 1
[0017] The synthetic method of organic synthesis intermediate 4-methylcyclohexanone comprises the steps:
[0018] A: Add 3mol 1-methoxy-4-methyl-6-bromocyclohexane in the reaction vessel, 900ml of sulfolane solution with a mass fraction of 20%, increase the solution temperature to 10°C, and add a mass fraction of 10% oxalic acid solution to adjust the pH of the solution to 5, then add 6mol potassium peroxodisulfate, and continue the reaction for 90min;
[0019] B: Then raise the temperature to 20°C, add 6mol mass fraction of 15% potassium chloride solution, 6mol phenylmercury acetate, continue the reaction for 1h, separate the layers of the solution, separate the oil layer, and use a 50% mass fraction of sodium nitrate solution Wash for 30 minutes, wash with 80% hexanoic acid solution for 20 minutes, recrystallize in 85% cyclohexanone solution, and dehydrate with anhydrous sodium sulfate to obtain 329.952 g of finished product 4-methylcyclohexanone. The rate is 98.2%.
Embodiment 2
[0021] The synthetic method of organic synthesis intermediate 4-methylcyclohexanone comprises the steps:
[0022] A: Add 3mol 1-methoxy-4-methyl-6-bromocyclohexane in the reaction vessel, 900ml of sulfolane solution with a mass fraction of 23.5%, increase the solution temperature to 12.5°C, and add a mass fraction of 13% oxalic acid solution to adjust the pH of the solution to 5.25, then add 7mol potassium peroxodisulfate, and continue the reaction for 110min;
[0023] B: Then raise the temperature to 22°C, add 7mol mass fraction of 18.5% potassium chloride solution, 7mol phenylmercury acetate, continue the reaction for 1.5h, separate the layers of the solution, separate the oil layer, and use a mass fraction of 53% sodium nitrate The solution was washed for 40 minutes, and the hexanoic acid solution with a mass fraction of 83% was washed for 30 minutes, recrystallized in a cyclohexanone solution with a mass fraction of 88.5%, and dehydrated with anhydrous sodium sulfate as a ...
Embodiment 3
[0025] The synthetic method of organic synthesis intermediate 4-methylcyclohexanone comprises the steps:
[0026] A: Add 3mol 1-methoxy-4-methyl-6-bromocyclohexane in the reaction vessel, 900ml of sulfolane solution with a mass fraction of 27%, increase the solution temperature to 15°C, and add a mass fraction of 16% oxalic acid solution to adjust the pH of the solution to 5.5, then add 8mol potassium peroxodisulfate, and continue the reaction for 130min;
[0027] B: Then raise the temperature to 24°C, add 8 mol of potassium chloride solution with a mass fraction of 22%, and 8 mol of phenylmercury acetate, continue to react for 2 hours, separate the layers of the solution, separate the oil layer, and use a 56% sodium nitrate solution Wash for 50 minutes, wash with 86% hexanoic acid solution for 40 minutes, recrystallize in a 92% cyclohexanone solution, and dehydrate with anhydrous sodium sulfate to obtain 331.632 g of finished product 4-methylcyclohexanone. The rate is 98.7%....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com