THR1442/L-aspartic acid co-crystal, and preparation method thereof and pharmaceutical composition thereof
A technology of aspartic acid and C1-C3, which is applied in the direction of drug combination, organic chemical method, cyanide reaction preparation, etc., can solve the problems of irregular particle shape, oil formation in water, high hygroscopicity, etc., and achieve good stability The effect of sex and mobility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
[0072] THR1442 was prepared according to the method described in Example [0110-0123] in the patent document CN102933592B.
[0073] 1 HNMR (500MHz, Methanol-d4): δ 7.38-7.27 (m, 3H), 7.13 (d, J = 8.3 Hz, 2H), 6.85 (d, J = 8.4 Hz, 2H), 4.13-4.03 (m, 5H), 3.91-3.87 (m, 1H), 3.87-3.83 (m, 2H), 3.71 (dd, J=11.9, 5.0 Hz, 1H), 3.50-3.40 (m, 4H), 3.30 (d, J= 9.1Hz, 1H), 0.59 (d, J=3.4Hz, 2H), 0.53-0.48 (m, 2H). Displayed as a known THR1442.
preparation example 2
[0075] The THR1442 diproline co-crystal was prepared by referring to the method described in Example 1F in the patent document CN102933592B.
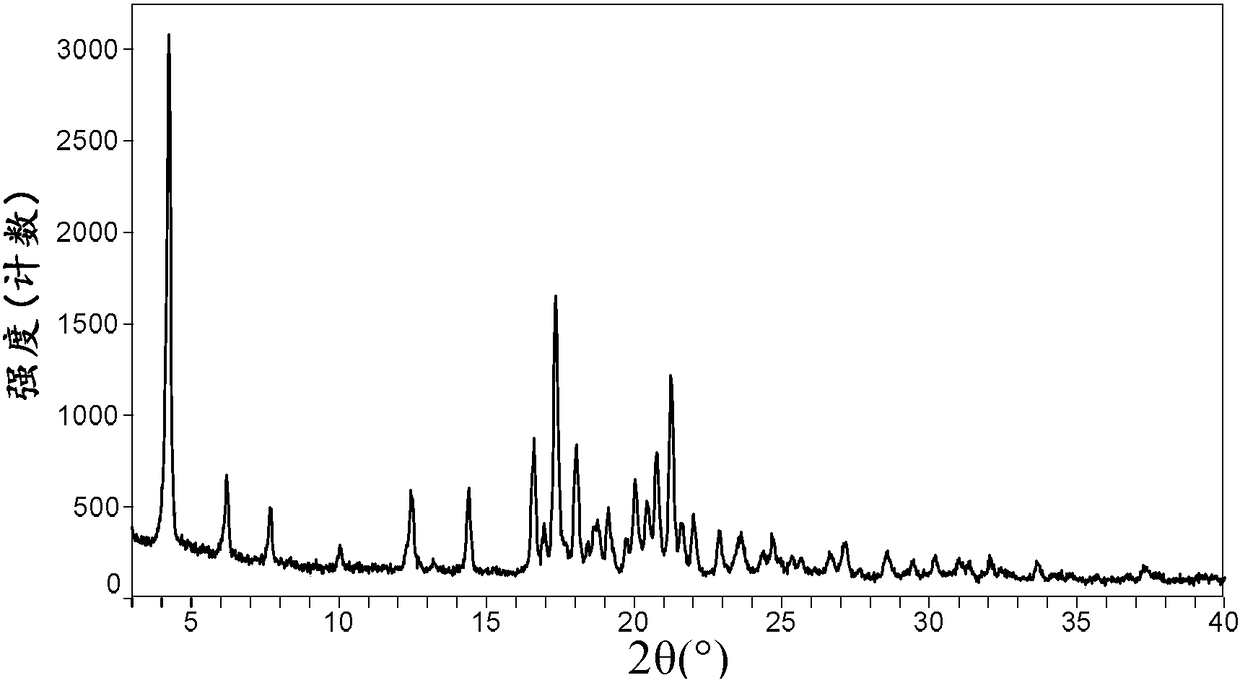
[0076] Its X-ray powder diffraction pattern is as follows figure 1 Shown.
[0077] Its PLM map is as follows figure 2 Shown.
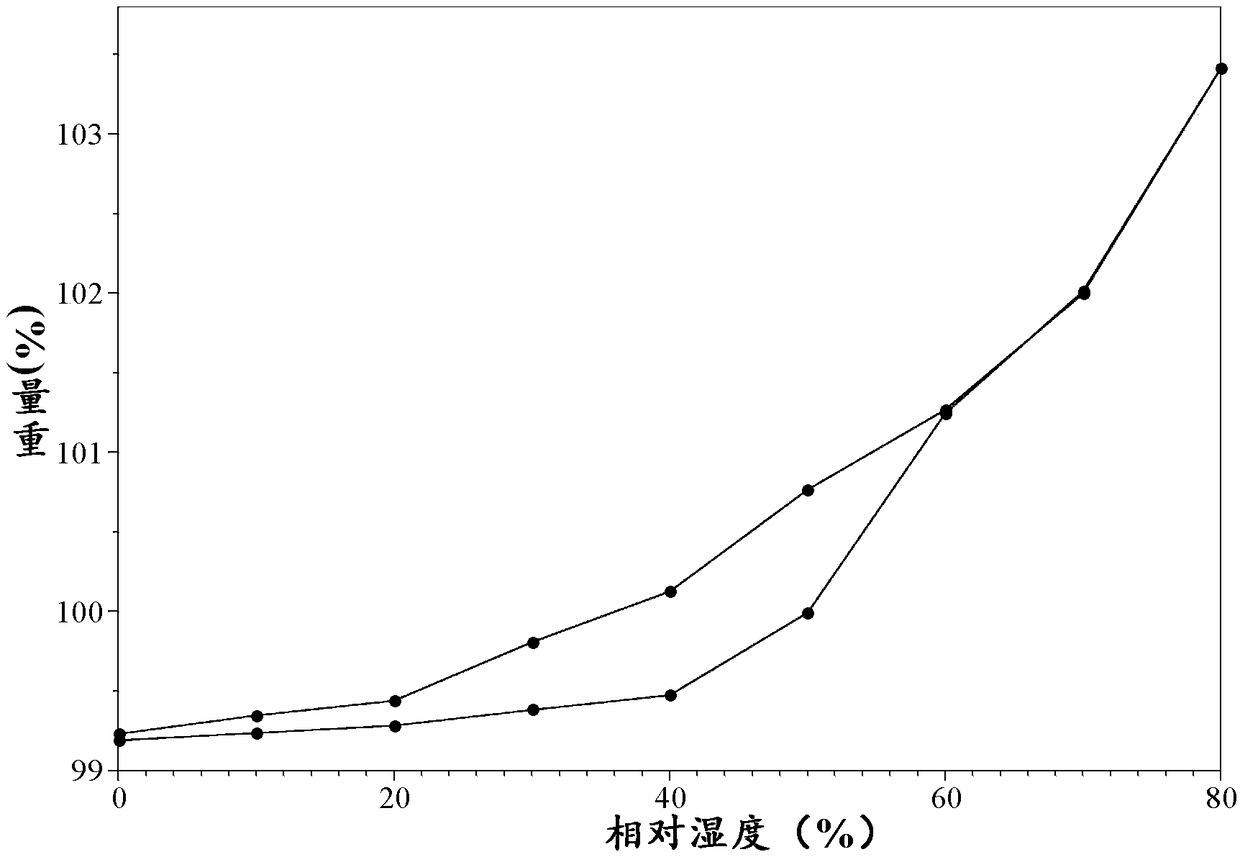
[0078] Its isotherm adsorption spectrum is as follows image 3 Shown.
Embodiment 1
[0080] Take 40 mg of THR1442 solid sample obtained in Preparation Example 1, add 0.2 mL of ethanol to form a clear solution, take 11.4 mg of L-aspartic acid, add 4 mL of water and ultrasound to form a clear solution, add the L-aspartic acid aqueous solution to the ethanol of THR1442 White turbidity precipitated in the solution. After stirring for 5 days at room temperature, it was filtered under reduced pressure and the solid was dried under vacuum at 40° C. for 10 hours to obtain 47.3 mg of THR1442L-aspartic acid co-crystal with a yield of 92%.
[0081] Its X-ray powder diffraction pattern is as follows Figure 4 Shown.
[0082] Its DSC spectrum is as Figure 5 Shown.
[0083] Its IR spectrum is as Image 6 Shown.
[0084] Its isotherm adsorption spectrum is as Figure 7 Shown.
[0085] Its PLM map is as follows Picture 8 Shown.
[0086] The proton nuclear magnetic spectrum data is: 1 HNMR (500MHz, DMSO-d6): δ7.33-7.22 (m, 2H), 7.16 (dd, J=8.2, 2.1Hz, 1H), 7.02 (d, J=8.5Hz, 2H), 6.77 ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com