D-psicose-3-epimerase mutant with improved catalytic activity and application of mutant
A technology of epimerase and psicose, applied in racemase/epimerase, isomerase, application, etc., can solve the problem of poor catalytic activity and limit the large scale of D-psicose Industrial production, high application costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
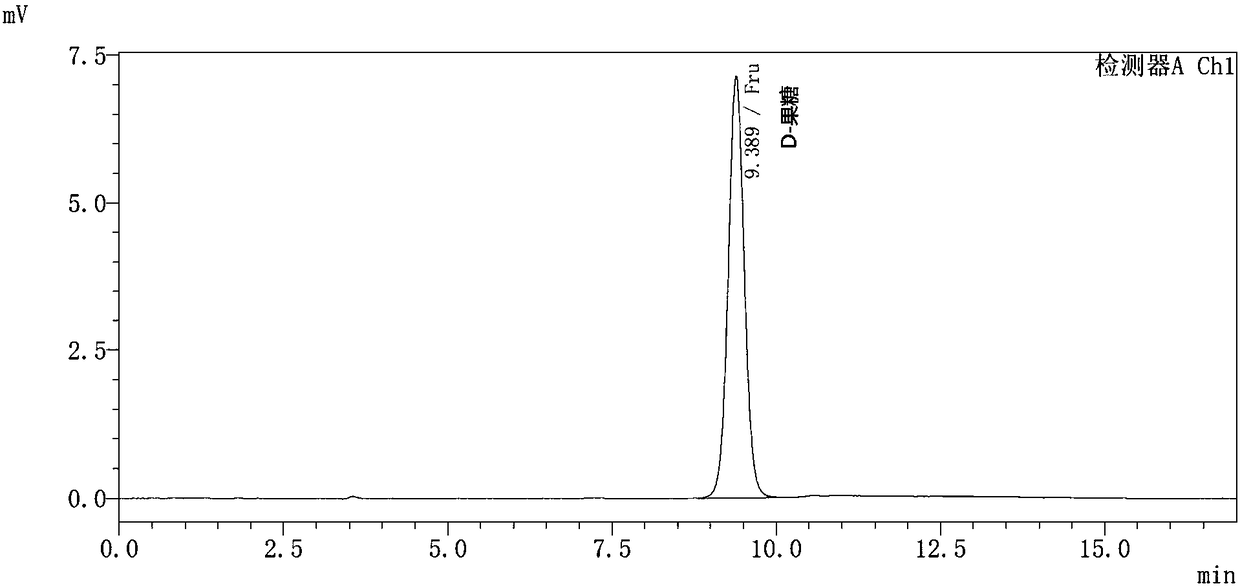
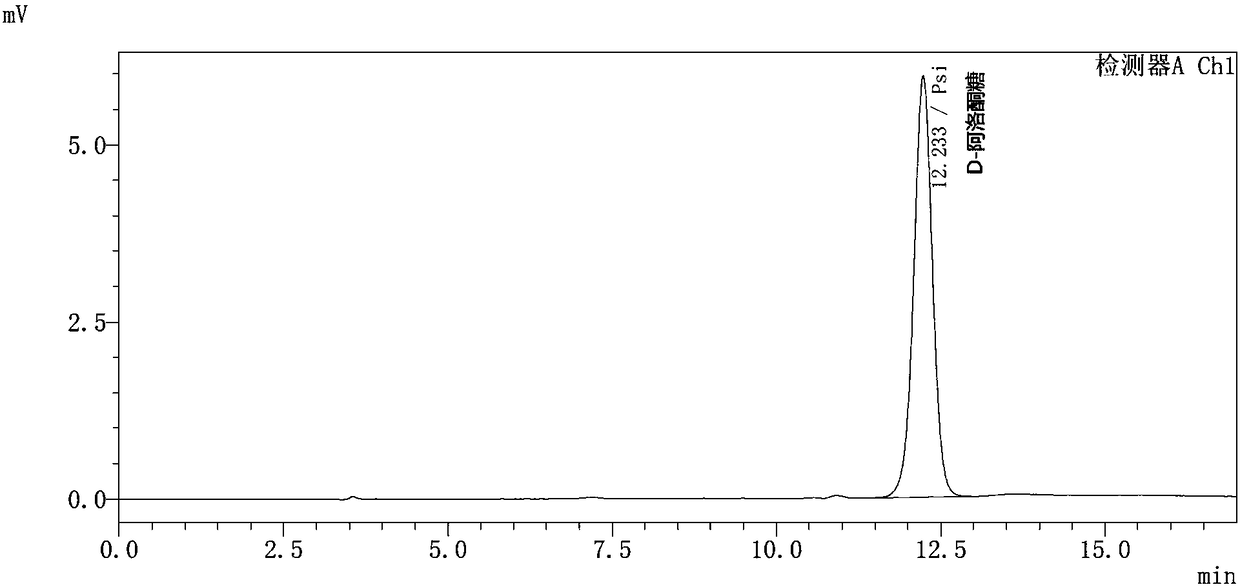
[0106] The preparation method of allulose
[0107] The present invention also provides a method for preparing allulose, the method comprising the steps of:
[0108] (1) contacting the mutated D-psicose-3-epimerase of the present invention with a reaction substrate to carry out a catalytic reaction, thereby generating the allulose;
[0109] (2) Optionally, isolating and purifying the psicose.
[0110] The present invention also relates to the application of the D-psicose prepared by the above method in the production of human food, animal feed, cosmetics or medicines.
[0111] The main advantages of the present invention include:
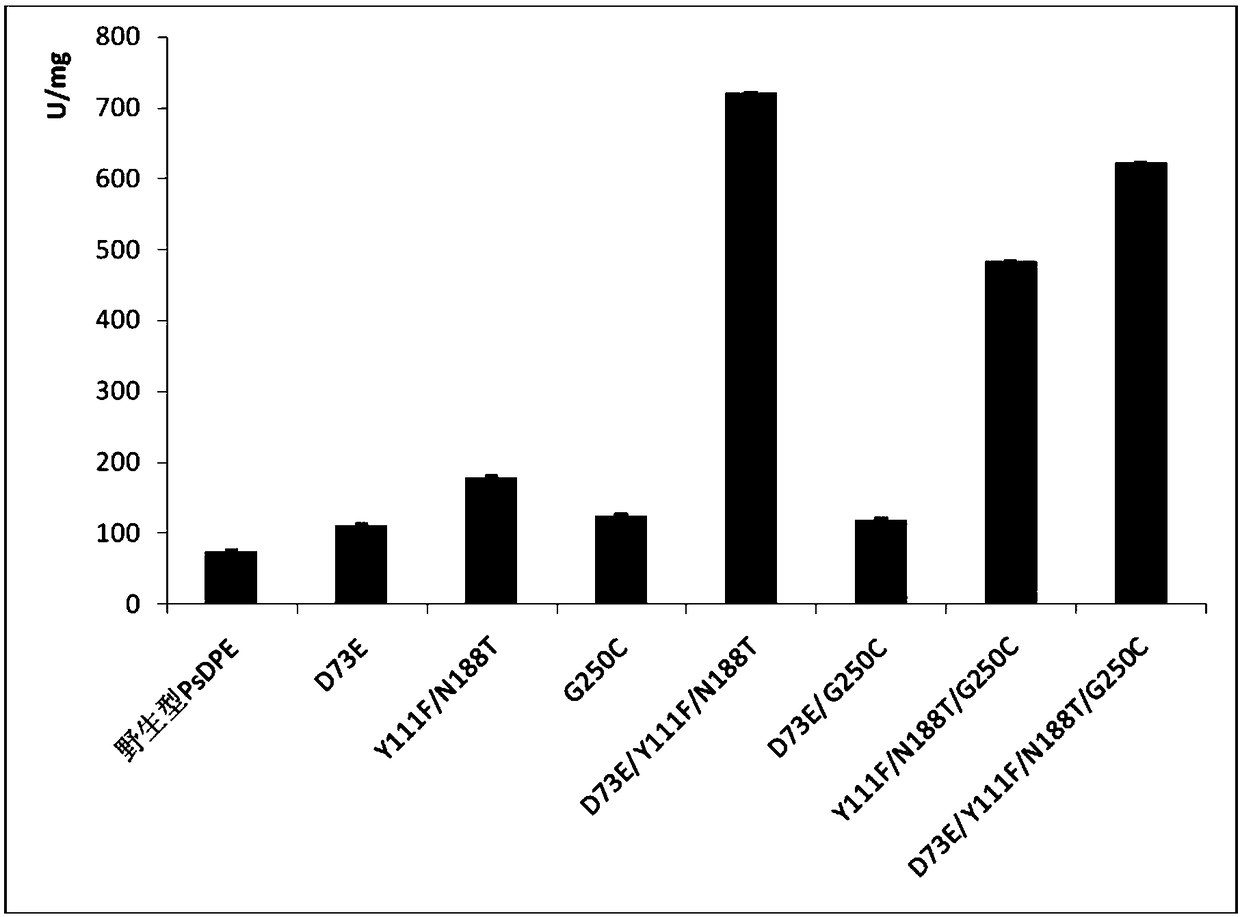
[0112] (1) The present invention provides a DPE enzyme mutant whose catalytic activity is significantly improved, and its catalytic activity (unit enzyme activity) is that of wild-type D-psicose-3-epimerase under the same conditions The catalytic activity is 9.6 times, greatly reducing the amount of enzymes used in the process of synthesizing D-ps...
Embodiment 1D
[0120] Example 1D-Establishment of a mutant library of psicose-3-epimerase
[0121] According to the BLAST comparison of the reported D-Psicose-3-epimerase (DPE) sequence, it was found that the amino acid sequence of an unknown protein derived from Paenibacillus senegalensis was consistent with other The similarity of several DPE genes is relatively large, and it is speculated that this gene has the ability to convert D-fructose into D-psicose, and it is a potential DPE gene. After the DNA sequence was optimized, the whole gene was synthesized by Changzhou Jiyu Biotechnology Co., Ltd., and recombined into the pET29a(+) vector (Novagen); after expression experiments and functional experiments, the protein can convert D-fructose into D - Allulose, which has the function of D-psicose-3-epimerase, belongs to this family, and its amino acid sequence is shown in SEQ ID NO.:1. Subsequently, protein directed evolution research was carried out on the basis of the wild-type protein.
...
Embodiment 2
[0130] Activation and induced expression of embodiment 2 mutant library
[0131] Pick a single clone from the above-mentioned cultured overnight plate to the LB liquid medium (ingredients: tryptone (Tryptone) 10g / L, yeast extract (Yeast Extract) ) 5g / L, sodium chloride 10g / L, wherein tryptone (Tryptone) and yeast extract (Yeast Extract) were purchased from Oxoid, and sodium chloride was purchased from Sinopharm Chemical Reagent Co., Ltd.) in 96 deep-well plates, in Culture overnight at 37°C with shaking at 220rpm. On the next day, draw 100 μl of the culture solution from the 96 empty plate cultured overnight, and add it to a fresh 96 deep-well plate containing LB liquid medium with a final concentration of 50 μg / ml kanamycin sulfate, at 37°C, 220rpm After 4 hours of shaking culture, IPTG with a final concentration of 1 mM was added for induction, and then culture was continued at 30° C. for 20 hours. A total of 400 single clones were picked for activity screening.
[0132] ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com