Anti-Ebola virus vp40 protein monoclonal antibody g7a6 and its application
A monoclonal antibody, Ebola virus technology, applied in antiviral immunoglobulin, instrument, biological material analysis, etc., to achieve significant antiviral effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
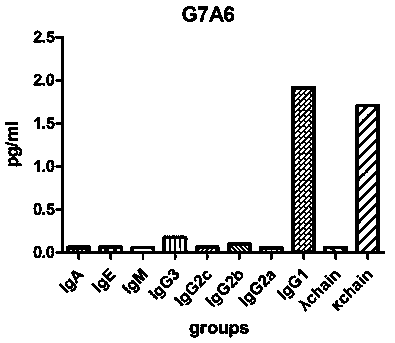
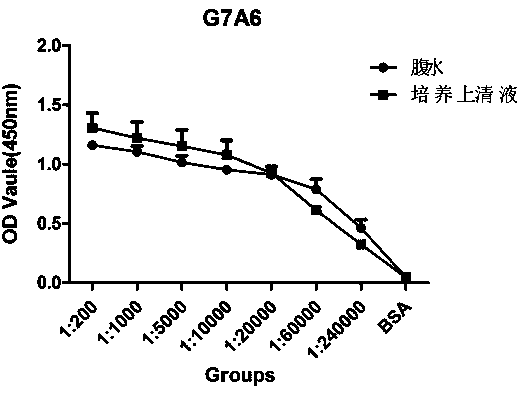
[0028] Example 1. Preparation of monoclonal antibody G7A6 against Ebola VP40 protein
[0029] (1) Immunization of mice: For the first immunization, 14 peptides of Ebola VP40 protein cross-linked to KLH (CTGKKVTSKNGQPI) and QuickAntibody-mouse5W were mixed 1:1 by volume, and the total volume was 100ul. Each BALB / C mouse 0.1ml (Ebola VP40 protein antigen peptide 100ug), intramuscular injection of the inner thigh. On the 21st day, boost the immunization in the same way. On the 35th day, a small amount of tail blood was collected for ELISA assay, and the antibody titer reached 1:32000. Immediately, the tail vein was injected to boost the immunization once, and cell fusion was carried out after 3 days.
[0030] (2) Culture of mouse myeloma cell SP2 / 0: The SP2 / 0 myeloma cell line from BALB / C mice was cultured and passaged in a medium containing 10% FBS-DMEM, and was cultured in a medium containing 5% CO. 2 Culture in a humidified 37°C incubator. Passage the day before fusion to e...
Embodiment 2
[0040] Example 2. Antiviral effect of monoclonal antibody G7A6 against Zaire Ebola VP40 protein, combined application of G7A6 with anti-GP monoclonal antibody (ZJEB8-01) and other anti-VP40 monoclonal antibodies (A2G7, F1B4) Research on viral effects
[0041] This experiment is realized by the following ways:
[0042] (1) Construction of Zaire Ebola virus-like particles (ZEBOV-trVLPs) in vitro replication model ( image 3 ). ZEBOV-trVLPs can simulate ZEBOV to synthesize miniature filovirus-like particles, which have basic functions such as invasion and replication, but are not biohazardous. Research is important.
[0043] (2) The trVLPs particles were collected by ultracentrifugation, and incubated with trVLPs (MIO=3) in a dose gradient of G7A6 monoclonal antibody (3, 5, 10, 15 μg / ml) for 1 h in vitro, and then added to a 293T cell culture plate (24-well). Plate); set the trVLPs without antibody as the control group. After 48 hours of incubation, the supernatant was disca...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com