Fluorescence labeled composite amplification kit capable of simultaneously amplifying human autosome and Y-chromosome STR (short tandem repeat) loci and application thereof
A Y chromosome and autosomal technology, which is applied to a fluorescent labeling compound amplification kit for simultaneously amplifying human autosomal and Y chromosome STR loci and its application fields, can solve the needs of case investigation that cannot meet the needs of investigation, the investigation ability is limited, and the Restrictions and other issues to achieve the effect of saving manpower, material and financial resources, shortening detection time, and high sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Example 1: Determination of detection loci; determination of kit primer set, amplification system, and amplification method.
[0041] 1. Determination of genetic loci
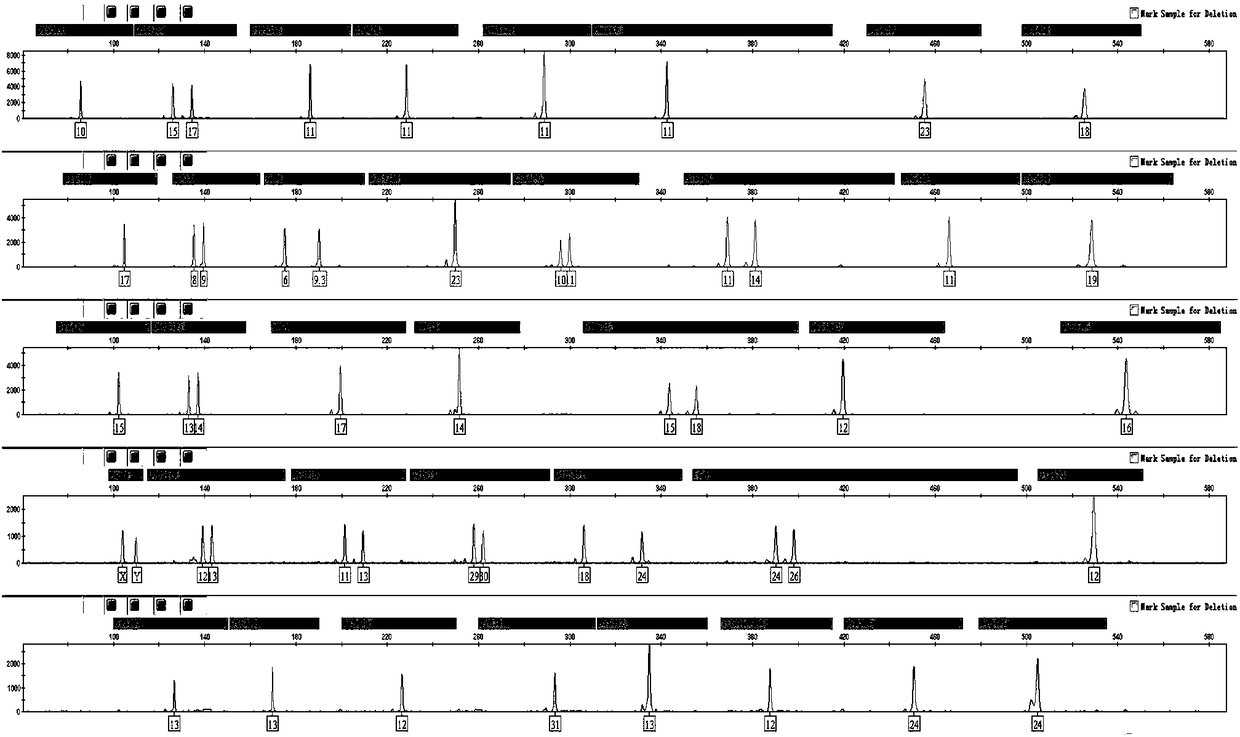
[0042] On the basis of the 201110243734.9 scheme, the present invention adds two autosomal loci D19S433 and PentaE, so that the number of autosomal STR loci reaches 18, and through genotype detection of more than 10,000 unrelated individuals, according to the alleles of each locus Distribution frequency statistical analysis of individual discrimination (DP), heterozygosity (H), non-parent exclusion rate (PE) and other data, the results show that the DP values of 16 A-STR loci are close to 0.9, and H is greater than 0.7. The PE value is above 0.5, which has good forensic application value. Although the genetic statistics of TH01 and TPOX loci are not as good as the above 16 A-STRs, they still belong to the recommended category of DNA database of the Ministry of Public Security. Maintaining the existi...
Embodiment 2
[0056] Example 2: Comparative test of novel fluorescent labels.
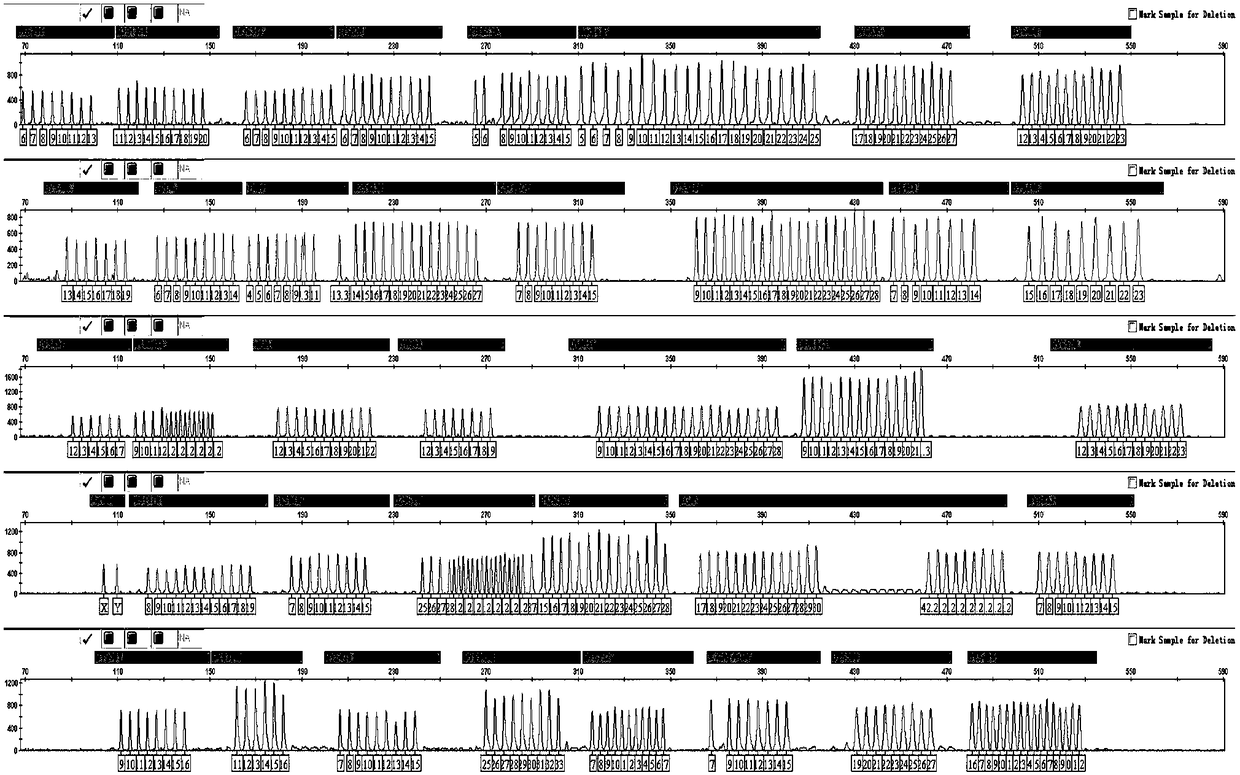
[0057] The present invention creatively extends the amplified fragment to 600bp in order to realize PCR single-tube amplification of 39 sites. For loci with larger amplified fragments (EA50 color DYS576, E11 color DYS533, 620 color DYS481), use traditional single fluorescent labeling, according to the technical scheme of Example 1, amplify a direct expansion blood card sample X1, and amplify the map See image 3 .
[0058] PCR is a complex molecular dynamics process. With the increase of the number of primers in the multiplex amplification system, the mutual interference between primers at different loci becomes more and more serious. At the same time, due to the larger amplification fragments and the longer fluorescence emission wavelength, the detection efficiency is lower. The locus of the fragment, using the traditional single fluorescent label, cannot meet the needs of amplification detection. The presen...
Embodiment 3
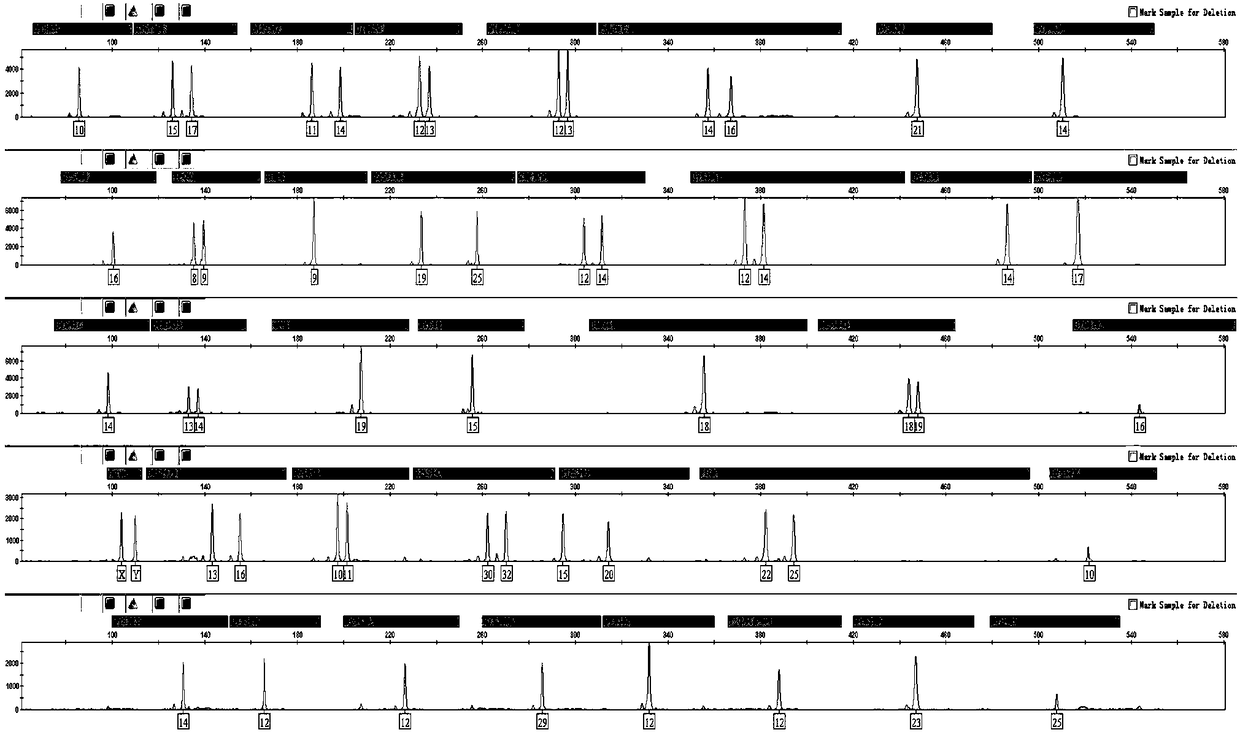
[0059] Example 3: Adjusting the PCR reaction program.
[0060] Because the kit of the present invention needs to meet the adaptability of different test materials and the tolerance of different annealing temperatures, it is necessary to test different amplification procedures to ensure that different test materials can obtain correct DNA analysis within a wide temperature range. type. First, the number of cycles test: 28 cycles, 29 cycles, 30 cycles, and 31 cycles were tested, and the experiment proved that the best cycle is 30; secondly, the annealing temperature test: 57 ° C, 58 ° C, 59 ° C were tested , 60°C, 61°C, 62°C, experiments have shown that the optimum temperature is 60°C and 58°C. The final amplification procedure was as follows:
[0061] Table 6 Amplification program
[0062]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com