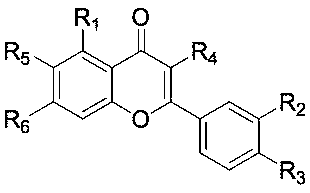
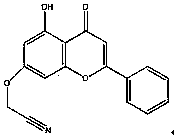
Flavonoid aromatase inhibitor and its preparation method and application
An aromatase and inhibitor technology, used in organic chemistry, anti-tumor drugs, drug combinations, etc., can solve problems such as low selectivity and poor efficacy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] 1. Synthesis and aromatase inhibitory activity of 2-(4-(7-(cyanomethyloxy)-4-oxo-4H-benzopyrone-3-yl)phenoxy)acetonitrile
[0046] 2. Preparation of 2-(4-(7-(cyanomethyloxy)-4-oxo-4H-benzopyrone-3-yl)phenoxy)acetonitrile
[0047] Take a dry 50mL round bottom flask, weigh 7-hydroxy-3-(4-hydroxyphenyl)-4H-benzopyran-4-one (didzein, 0.2543g, 1mmol) into it, and then add dropwise N,N'-Dimethylformamide (DMF, 3mL) was placed in a bottle, stirred at room temperature for 5min, then weighed sodium hydride (0.0480g, 2mmol) and added to the reaction solution, and continued to stir until no more Saturated with gas, then added chloroacetonitrile (503 µL, 4 mmol) dropwise at room temperature, added chloroacetonitrile (76 µL, 1 mmol) after 30 min of reaction, put the reaction bottle in an oil bath at 61 °C and continued to stir, then reacted for 13 hours , TLC monitoring, until the reaction of raw materials is complete. Transfer the reaction solution to a 500mL beaker, add 300mL of...
Embodiment 2
[0075] Synthesis and aromatase inhibitory activity of 2-(5-hydroxy-2-phenyl-4H-benzopyrone-7-oxy)acetonitrile
[0076] Preparation of 2-(5-hydroxy-2-phenyl-4H-benzopyrone-7-oxy)acetonitrile
[0077] Take a dry 50mL round bottom flask, weigh 5,7-dihydroxy-2-phenyl-4H-benzopyrone-4-one (chrysin, 0.2543g, 1 mmol) into it, and then drop N,N'-Dimethylformamide (DMF, 3mL) was placed in a bottle, stirred at room temperature for 5min, then weighed sodium hydride (0.0480g, 2mmol) and added to the reaction solution, and continued to stir until no more Saturated with gas, then added chloroacetonitrile (503 µL, 4 mmol) dropwise at room temperature, added chloroacetonitrile (76 µL, 1 mmol) after 30 min of reaction, put the reaction bottle in an oil bath at 61 °C and continued to stir, then reacted for 13 Hours, TLC monitoring, until the reaction of raw materials is complete. Transfer the reaction solution to a 500mL beaker, add 300mL of distilled water and stir with a glass rod, then add...
Embodiment 3
[0083] 2-((2-(4-(cyanomethyloxy)-3-hydroxyphenyl)-5-hydroxy-4-oxo-4H-benzopyrone-7-yl)oxy)acetonitrile synthesis and aromatase inhibitory activity
[0084] Preparation of 2-((2-(4-(cyanomethyloxy)-3-hydroxyphenyl)-5-hydroxy-4-oxo-4H-benzopyrone-7-yl)oxy)acetonitrile
[0085] Take a dry 50mL round bottom flask, weigh 2-(3,4-dihydroxyphenyl)-5,7-dihydroxy-4H-benzopyrone-4-one (luteolin, 0.2862g, 1 mmol) into it, then drop N,N'-dimethylformamide (DMF, 3mL) into the bottle, stir at room temperature for 5min, then weigh sodium hydride (0.0480g, 2mmol) into the reaction solution Continue to stir until no more gas comes out, then add chloroacetonitrile (503µL, 4mmol) dropwise at room temperature, add chloroacetonitrile (76µL, 1mmol) after 30min reaction, put the reaction bottle in a 61℃ Stirring was continued in the oil bath, followed by reaction for 13 hours, monitored by TLC, until the reaction of the raw materials was complete. Transfer the reaction solution to a 500mL beaker, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com