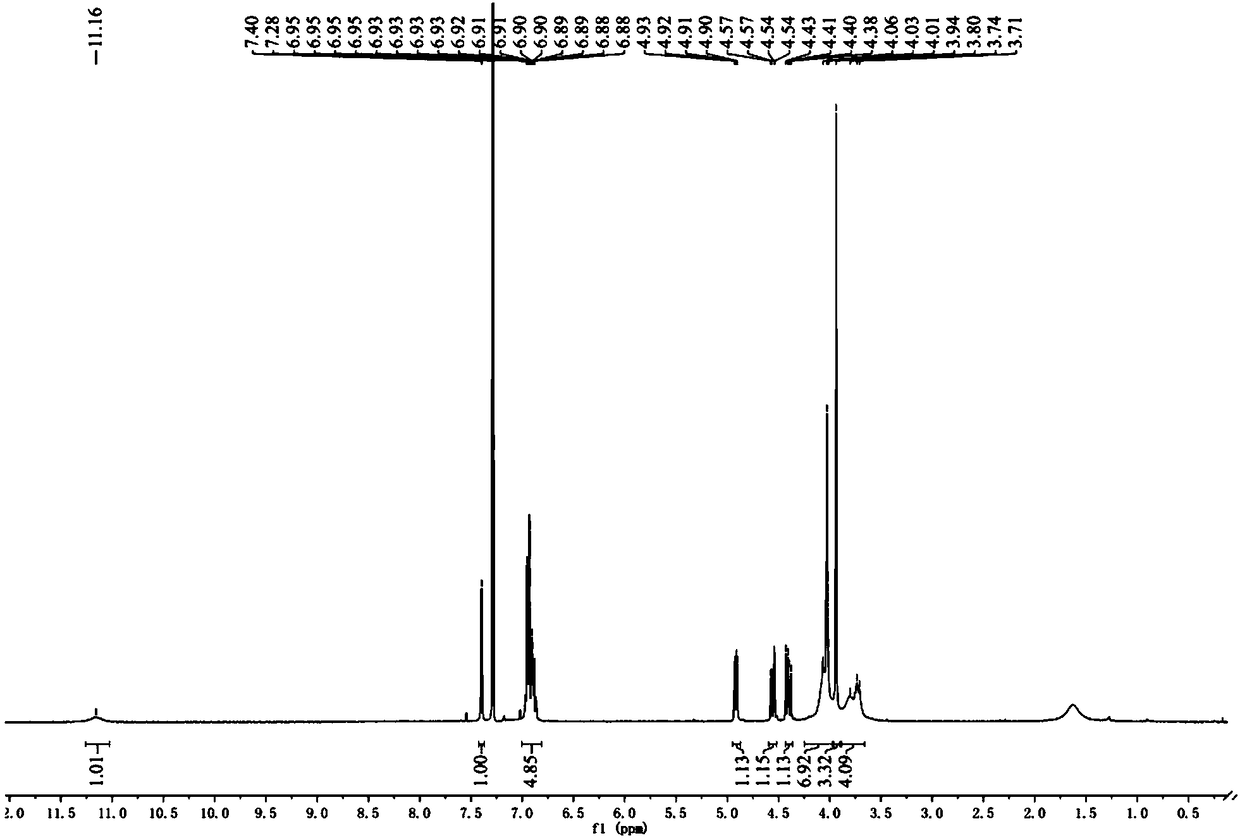
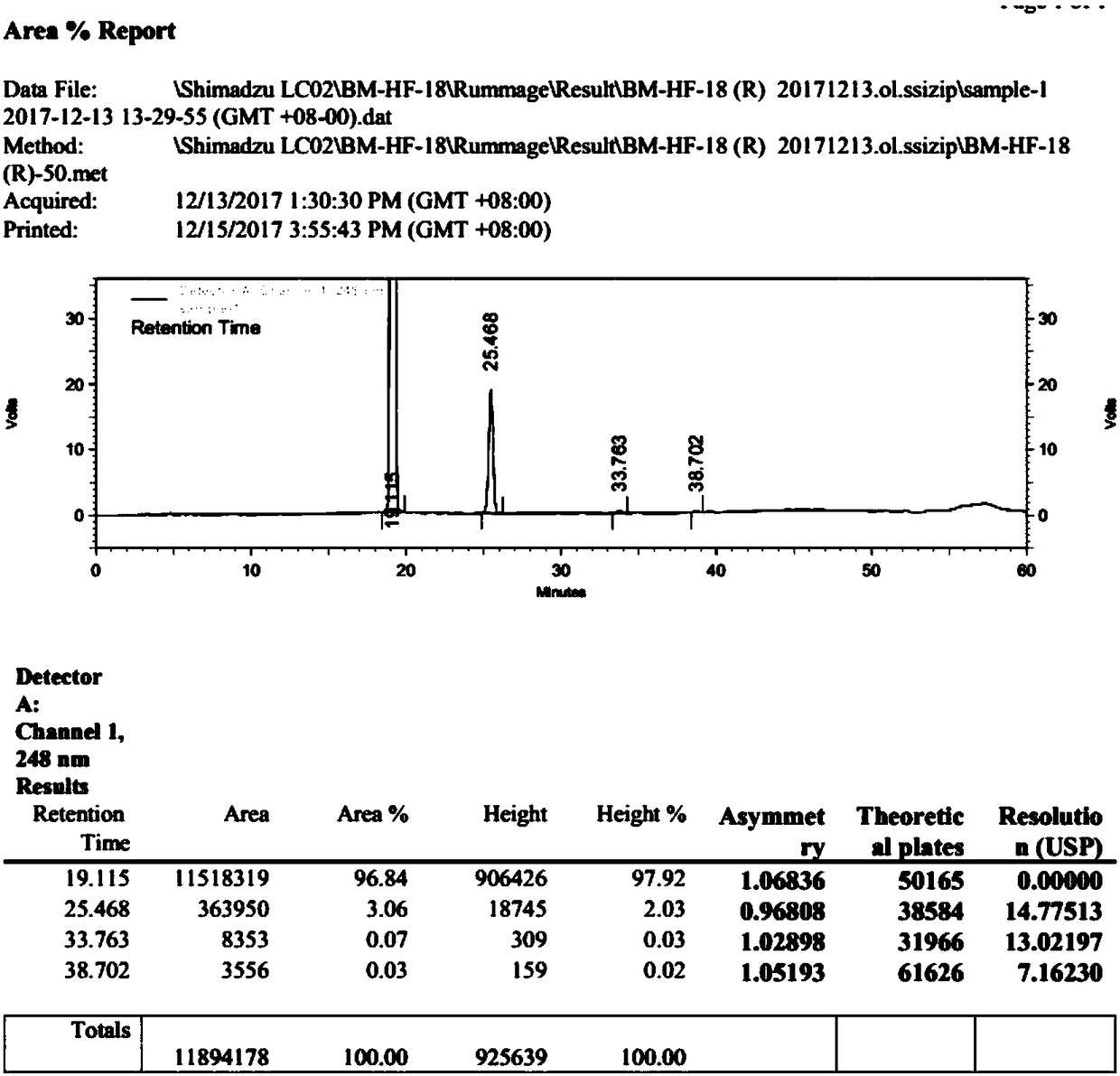
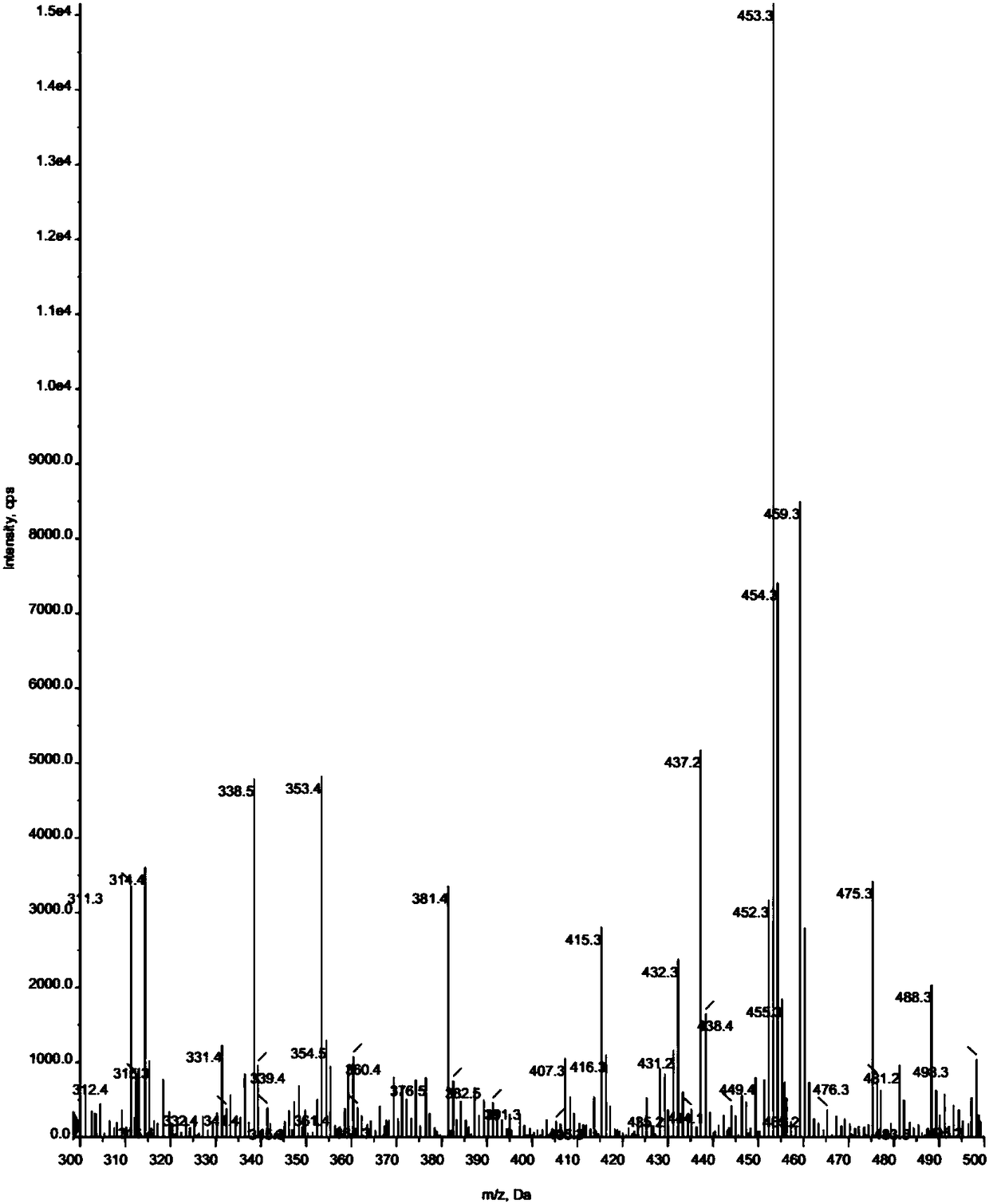
Synthetic method doxazosin impurity
A doxazosin and synthesis method technology, applied in the field of medicine, can solve the problems of poor qualitative and quantitative accuracy and high risk, and achieve the effects of low synthesis cost, short reaction route and low energy consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] A method for synthesizing doxazosin impurities, comprising the steps of: mixing 5ml of n-butanol, 0.24g of 2-chloro-4-hydroxyl-6,7-dimethoxyquinazoline, 0.28g of N-(1, 4-Benzodioxane-2-carbonyl)piperazine was added into the three-necked flask, heated to reflux, stirred for 1 h, cooled, filtered and dried at 50°C to obtain 0.42 g of doxazosin impurity with a yield of 95 %, wherein, the reaction equation of the above-mentioned doxazosin impurity preparation is as follows:
[0025]
Embodiment 2
[0027] A method for synthesizing doxazosin impurities, comprising the steps of: mixing 50ml of ethanol, 2.2g of 2-chloro-4-hydroxy-6,7-dimethoxyquinazoline, 2.8g of N-(1,4- Benzodioxane-2-carbonyl)piperazine was added to the three-necked flask, heated to 80°C, and stirred for 5 hours under reflux. After cooling, filtration and drying at 40°C, 3.73 g of doxazosin impurity was obtained with a yield of 90 %, wherein, the reaction equation of the above-mentioned doxazosin impurity preparation is as follows:
[0028]
Embodiment 3
[0030] A method for synthesizing doxazosin impurities, comprising the steps of: mixing 50ml of methanol, 2.4g of 2-chloro-4-hydroxy-6,7-dimethoxyquinazoline, 2.6g of N-(1,4- Benzodioxane-2-carbonyl)piperazine was added to the three-necked flask, heated to 70°C, stirred for 8 hours, cooled, filtered and dried at 60°C to obtain 3.57g of doxazosin impurities, with a yield of 85% , wherein, the reaction equation of the above-mentioned doxazosin impurity preparation is as follows:
[0031]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com