Piperacillin impurity and preparation method thereof
A piperacillin and impurity technology, applied in the field of medicine, can solve problems such as non-compliance, unknown impurity content cannot be effectively controlled, and product quality is affected
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Add 10g of finished piperacillin, 100ml of ethanol, 100ml of water, and 1.8g of sodium bicarbonate into a 500ml three-necked bottle, place the three-necked bottle in a water bath, control the temperature of the solution at 25-30°C, stir for 15 minutes, and the pH=7.97 solution Clear, no solids. After stirring for 25 hours, T=27°C, pH=8.67, start to add 2N HCl solution dropwise, control the dropping speed, after the dropwise addition is completed, T=28°C, pH=1.96, stir for 1 hour and then filter with suction, add 250ml of the solid into three ports In the bottle, add 100ml of water and raise the temperature to 80°C, stir for 30 minutes, cool down at 0°C and stir for 1 hour, then perform suction filtration and dry treatment. The impurity purity obtained by liquid phase detection of the obtained solid was 28.5%.
Embodiment 2
[0043] Add 10g of finished piperacillin, 100ml of ethanol, 100ml of water, and 2.0g of sodium bicarbonate into a 500ml three-necked bottle, place the three-necked bottle in a water bath, control the temperature of the solution at 30-35°C, stir for 15 minutes, and the pH=7.28 solution Clear, no solids. Under the condition of T=30-35°C, after stirring for 30 hours, T=31°C, pH=7.24, start to add 2N HCl solution dropwise, control the drop rate, after the dropwise addition, pH=2.48, stir for 1 hour and then suction filter , the obtained solid was added to a 250ml three-neck flask, 100ml of water was added and the temperature was raised to 80°C, and after stirring for 30 minutes, the temperature was lowered at 0°C and cooled and stirred for 1 hour, then dried by suction filtration. The impurity purity obtained by liquid phase detection of the obtained solid was 20%.
Embodiment 3
[0045] Add 10g of finished piperacillin, 100ml of ethanol, 100ml of water, and 1.8g of sodium bicarbonate into a 500ml three-necked bottle, place the three-necked bottle in a water bath, control the temperature of the solution at 25-30°C, stir for 15 minutes, and the pH=7.62 solution Clear, no solids. After stirring for 26 hours, T = 28°C, pH = 8.76, start to add 2N HCl solution dropwise, control the dropping speed, after the dropwise addition, T = 28°C, pH = 1.96, stir for 1 hour and then filter with suction, add the obtained solid to 250ml three ports In the bottle, add 100ml of water and stir at room temperature for 30 minutes, then cool and stir at about 0°C for one hour, then filter with suction for drying. The impurity purity obtained by liquid phase detection of the obtained solid was 25.6%.
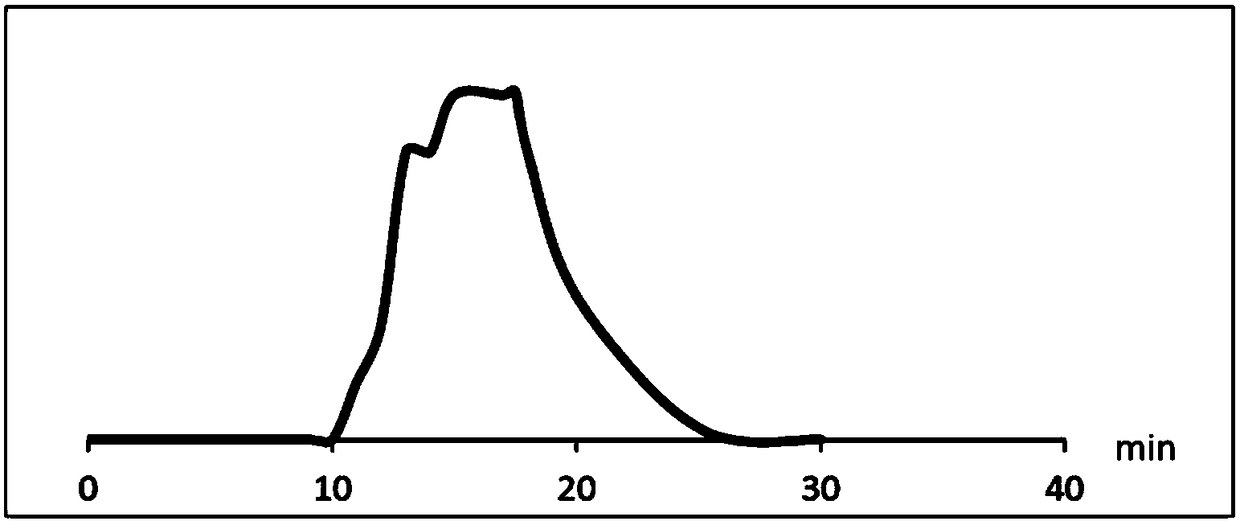
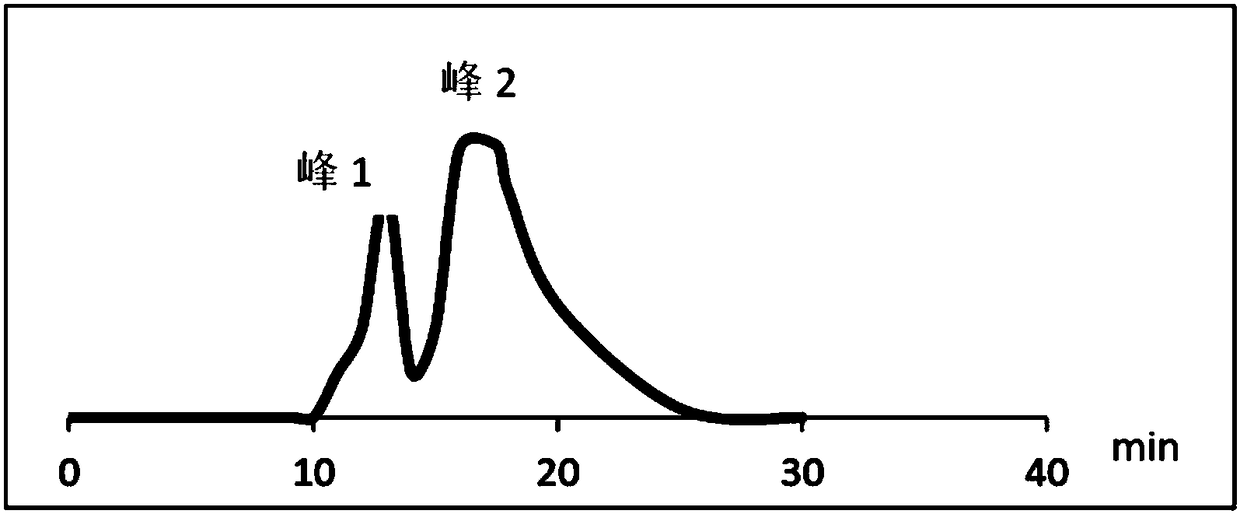
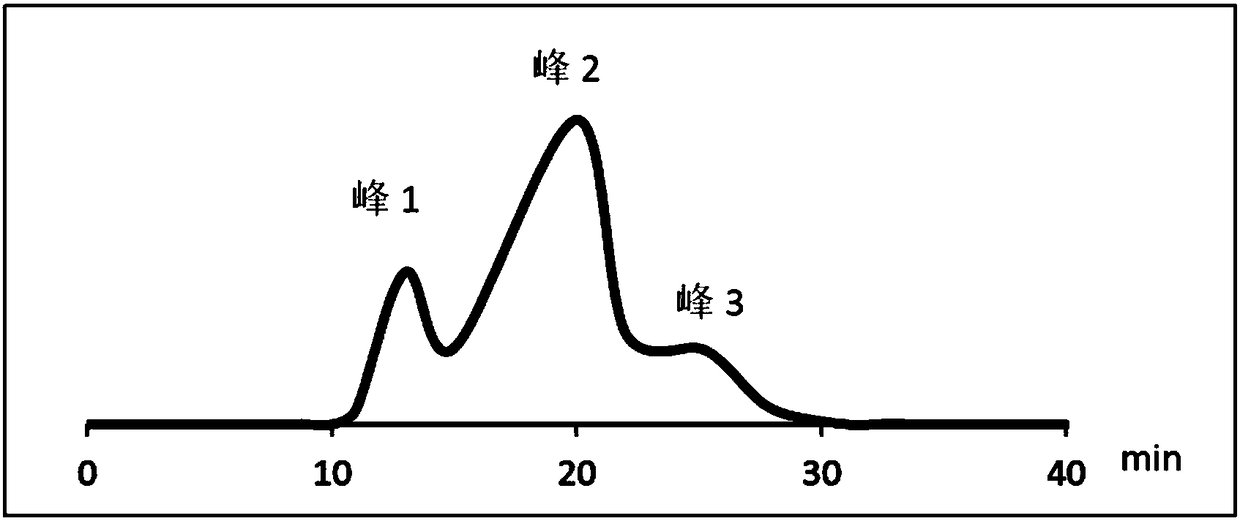
[0046] 2. Separation of impurities with RRT=1.75
[0047] The liquid chromatography conditions are as follows:
[0048] Chromatographic column: Agient ZORBAX SB-C18 liquid chroma...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com