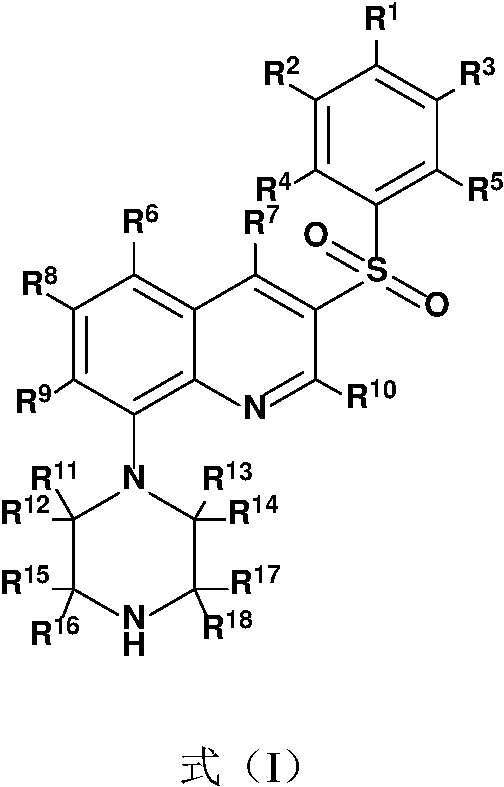
A kind of substituted quinoline compound and its pharmaceutical composition
A compound and composition technology, applied in the field of medicine, can solve the problems of cholinesterase inhibitor side effects, unsatisfactory curative effect and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
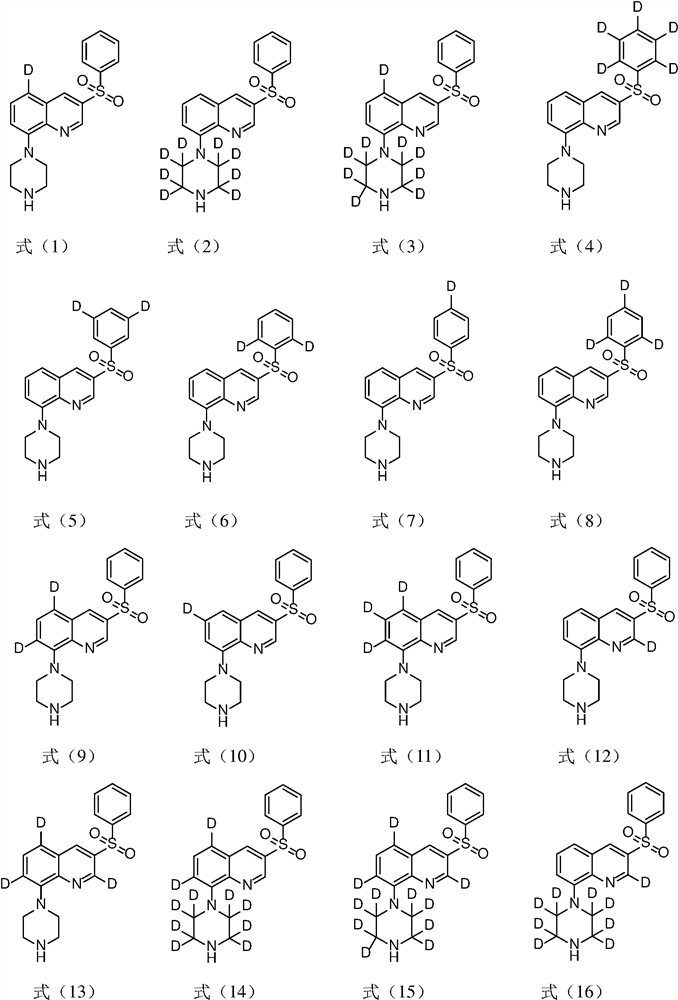
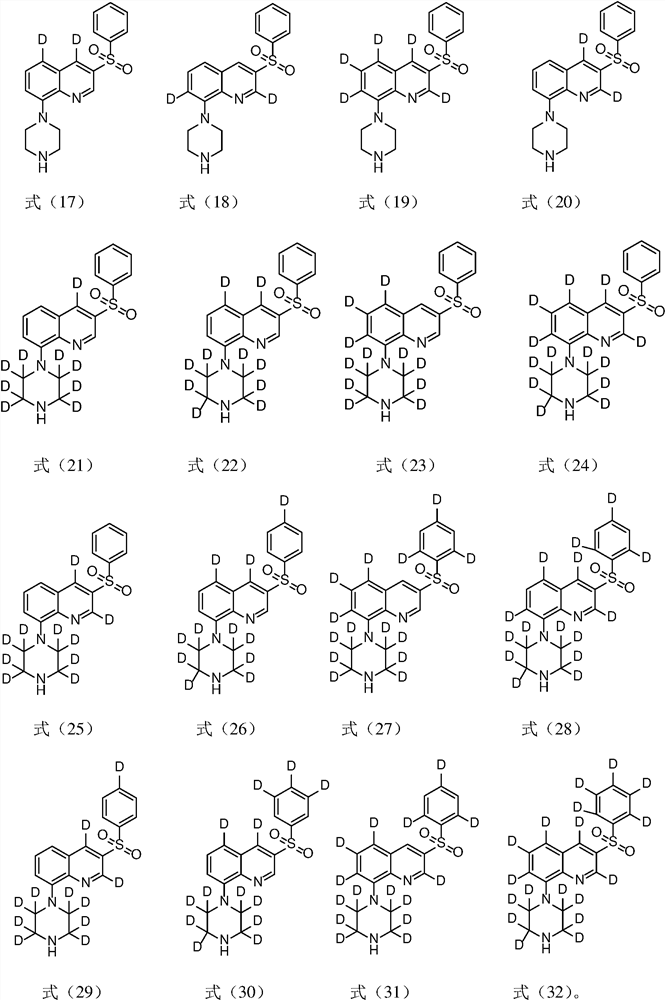
[0046] Example 1 Preparation of 5-d-3-benzenesulfonyl-8-piperazin-1-yl-quinoline (compound 10)
[0047]
[0048] Concrete synthetic steps are as follows:
[0049]
[0050] Step 1: Synthesis of compound 3.
[0051] Under nitrogen protection, N-iodosuccinimide (NIS, 6.88g, 30.58mmol) was added to the acetic acid solution (20mL) of 8-fluoroquinoline (compound 1, 3.0g, 20.39mmol), heated to 80°C and stirred overnight at this temperature. Add sodium sulfite (1.5g) and stir for 1 hour, then add iodine to quench the reaction, cool to room temperature after 1 hour, filter under low pressure, wash the crystals with acetic acid / water (1 / 2), and dry to obtain a yellow solid product Compound 3 3.5 g, the yield is 69%. LC-MS(APCI): m / z=293.9(M+1) + . 1 H NMR (300MHz, CDCl 3 ) (δ / ppm) 9.08 (d, J = 1.8 Hz, 1H), 8.58 (t, J = 1.8 Hz, 1H), 7.52-7.43 (m, 3H).
[0052] Step 2: Synthesis of Compound 5.
[0053] Under the protection of nitrogen, compound 4 (219mg, 2.49mmol) and cupro...
Embodiment 2
[0062] Example 2 Preparation of 3-benzenesulfonyl-8-(2,2,3,3,5,5,6,6-d8 piperazine)-1-yl-quinoline (compound 12)
[0063]
[0064] Concrete synthetic steps are as follows:
[0065]
[0066] Step 1: Synthesis of Compound 12.
[0067] Compound 5 (170 mg, 452 μmol) and potassium carbonate (38 mg, 3.17 mmol) were dissolved in 5 mL of n-propanol (n-PrOH), compound 11 (278 mg, 2.26 mmol) was added to the solution, heated to 95 ° C and Reacted for 17 hours, removed the solvent, added water and extracted with ethyl acetate, collected the organic phase and purified to obtain 25 mg of compound 12 as a yellow solid product with a yield of 15%. LC-MS(APCI): m / z=362.2(M+1) + . 1 H NMR (300MHz, CDCl 3 )(δ / ppm) 9.23(d, J=2.4Hz, 1H), 8.81(t, J=2.4Hz, 1H), 8.06-8.03(m, 2H), 7.65-7.56(m, 5H), 7.32- 7.29 (m, 1H).
Embodiment 3
[0068] Example 3 Preparation of 3-benzenesulfonyl-8-piperazin-1-yl-5,7-d2-quinoline (compound 13)
[0069]
[0070] Concrete synthetic steps are as follows:
[0071]
[0072] Step 1: Synthesis of Compound 13.
[0073] Compound 7 (130mg, 287μmol) was dissolved in deuterium water (5mL), deuterated hydrochloric acid (24μL, 287μmol, 12M / L) was added dropwise, heated to 180°C and microwaved for 2 hours, cooled to room temperature, poured into saturated Sodium bicarbonate solution was extracted with dichloromethane, and the organic phase was collected and purified by column to obtain 30 mg of compound 13 as a yellow solid product with a yield of 30%. LC-MS(APCI): m / z=353.3(M+1) + . 1 H NMR (300MHz, CDCl 3 )(δ / ppm) 9.22(d, J=2.4Hz, 1H), 9.05(d, J=2.4Hz, 1H), 8.10-8.07(m, 2H), 7.72-7.62(m, 4H), 3.25( t,J=4.5Hz, 4H), 2.96(t,J=4.5Hz, 4H).
PUM
| Property | Measurement | Unit |
|---|---|---|
| Weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com