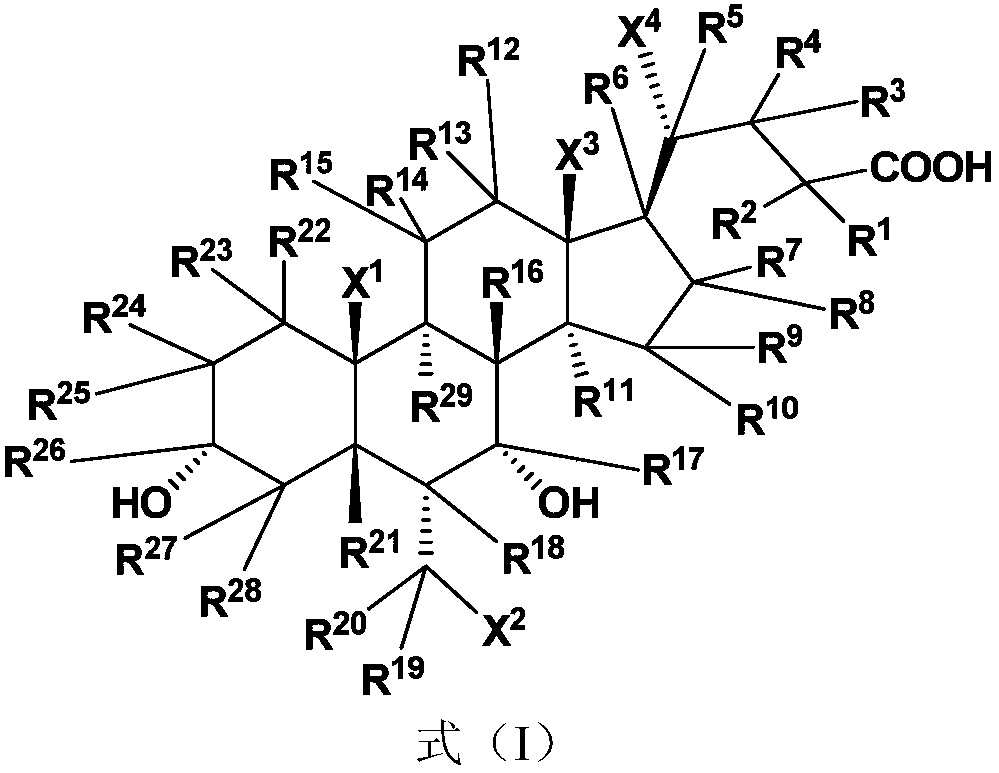
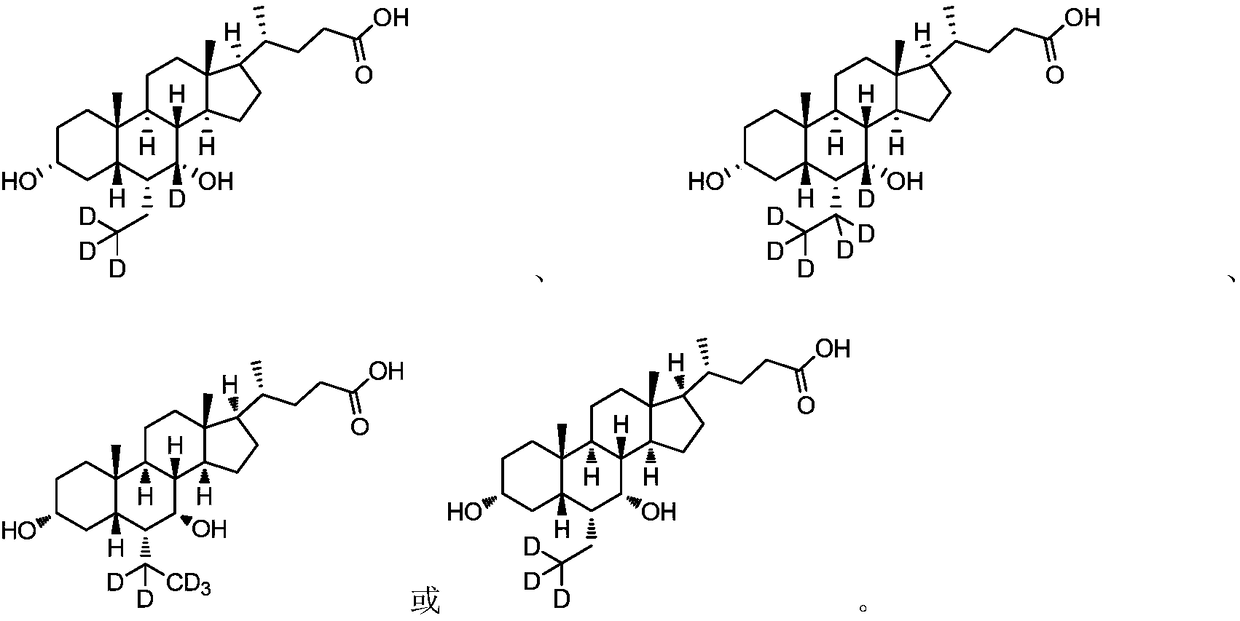
Cholanic acid compounds for preventing or treating fxr-mediated diseases
A compound, cholanoic acid technology, applied in the field of medicine, can solve problems such as increasing energy consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
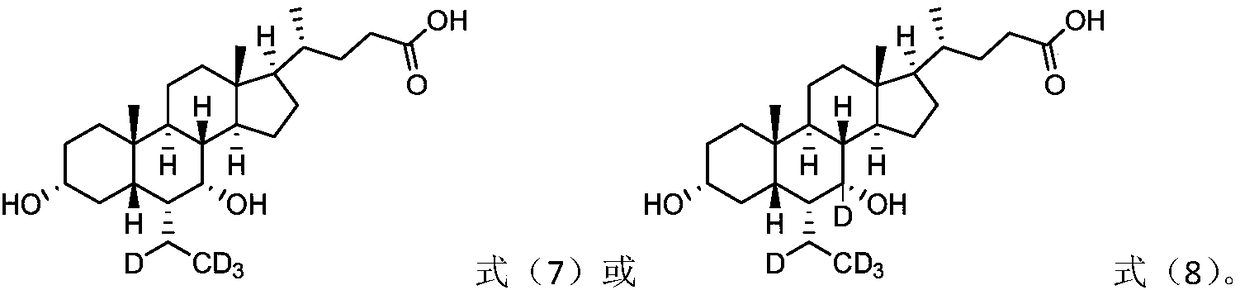
[0061] Example 1 Preparation of 3α, 7α-di-hydroxyl-7-d-6α-ethyl-5β-cholanic acid (compound 7)
[0062]
[0063] Concrete synthetic steps are as follows:
[0064]
[0065]
[0066] Step 1: Synthesis of methyl α-hydroxy-7-keto-5β-cholanate (compound 2).
[0067] Two drops of concentrated sulfuric acid were added dropwise to 3α-hydroxy-7-keto-5β-cholanic acid (5.00 g, 12.80 mmol) in methanol, and reflux reaction (65° C.) for 3 hrs. Concentrate under reduced pressure to remove methanol, concentrate liquid column chromatography to obtain 4.60 g colorless solid (compound 2), yield: 88.3%.
[0068] LC-MS(APCI):m / z=405.3[M+1] + .
[0069] Step 2: Synthesis of methyl α-7α-di-trimethylsilyloxy-5β-cholanate (compound 3).
[0070] At -78°C, trimethylchlorosilane (7.15 mL, 57.0 mmol) was added dropwise to a solution of lithium diisopropylamide (LDA 2M, 34.2 mL, 68.4 mmol) in anhydrous tetrahydrofuran (70 mL), and stirred for 30 minutes. Then a mixture of 3α-hydroxy-7-keto-5β...
Embodiment 2
[0083] Example 2 Preparation of 3α, 7α-di-hydroxyl-6α-(ethyl-1-d)-5β-cholanic acid (compound 10)
[0084]
[0085] Concrete synthetic steps are as follows:
[0086]
[0087] Step 1: Synthesis of methyl 3α-hydroxy-6α-(ethyl-1-d)-6-d-7-keto-5β-cholanate (compound 8).
[0088] Pd / C (10%, 55% in D 2 2, 60 mg) was added to 3α-hydroxy-6-ethylidene-7-keto-5β-cholanoic acid methyl ester (600 mg, 1.39 mmol) in anhydrous tetrahydrofuran and deuterated methanol (20 mL, 1:1 v / v ) in a mixed solvent. The air was replaced by deuterium gas, and the reaction was stirred overnight under deuterium gas. Filtrate with celite, concentrate under reduced pressure, and purify by column chromatography to obtain 420 mg of light yellow oil, yield: 70.0%.
[0089] LC-MS(APCI):m / z=435.3[M+1] + .
[0090] Step 2: Synthesis of 3α-hydroxy-6α-(ethyl-1-d)-7-keto-5β-cholanic acid (Compound 9).
[0091] Sodium hydroxide (120mg, 2.90mmol) was added to 3α-hydroxy-6α-(ethyl-1-d)-6-d-7-keto-5β-cholano...
Embodiment 3
[0096] Example 3 Preparation of 3α, 7α-di-hydroxyl-6α-ethyl-6-d-5β-cholanic acid (compound 12)
[0097]
[0098] Concrete synthetic steps are as follows:
[0099]
[0100] Step 1: Synthesis of 3α-hydroxy-6α-ethyl-6-d-7-keto-5β-cholanic acid (Compound 11).
[0101] Sodium deuterated oxide (120 mg, 2.90 mmol) was added to methyl 3α-hydroxy-6α-ethyl-7-keto-5β-cholanate (420 mg, 0.97 mmol) in deuterated methanol and heavy water (20 mL, 1: 1v / v) in a mixed solvent, the reaction was stirred overnight, methanol was removed under reduced pressure, diluted with water (20mL), acidified with 2N HCl, extracted with ethyl acetate (50mL x3), the organic layer was washed with saturated brine, anhydrous sulfuric acid Sodium dry. The organic layer was concentrated under reduced pressure, and the concentrate was purified by column chromatography to obtain 280 mg of white solid, yield: 68.8%.
[0102] LC-MS(APCI):m / z=418.1[M-1] - .
[0103] Step 3: Synthesis of 3α,7α-di-hydroxy-6α-(...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com