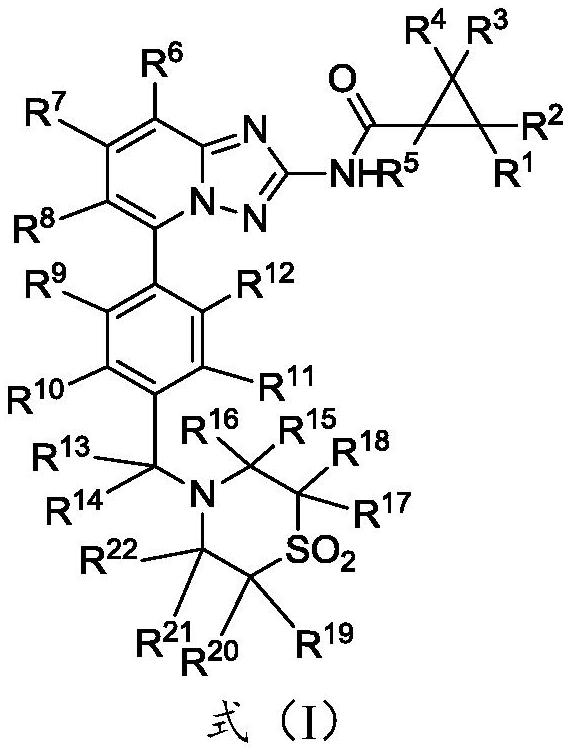
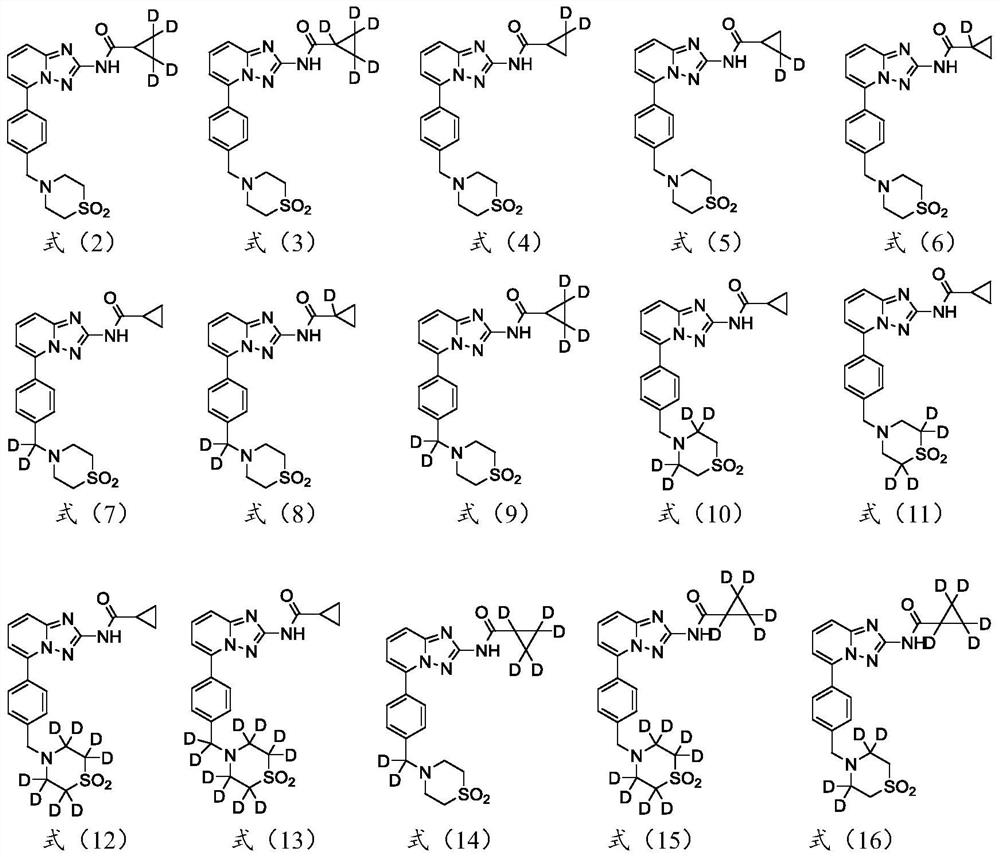
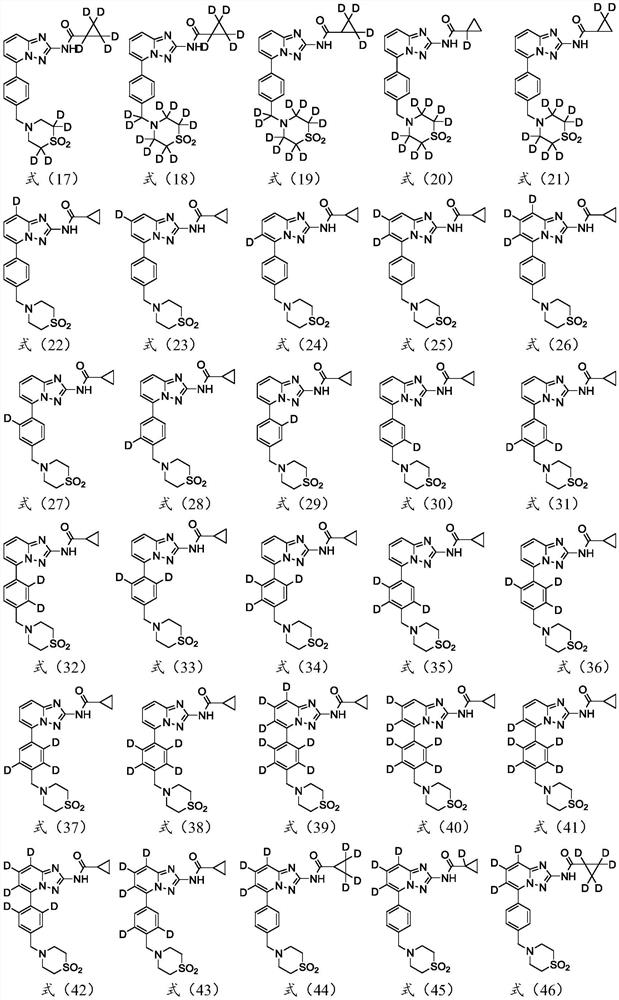
A kind of substituted pyridine amide compound and application thereof
A compound and composition technology, applied in the field of medicine, can solve problems such as inability to reduce pain
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0159] Example 1 N-(5-(4-((1,1-dioxo-4-thiomorpholine-2,2,6,6-d4)methyl)phenyl)-[1,2, 4] Triazolo[1,5-a]pyridin-2-yl)cyclopropanylcarboxamide (compound 13)
[0160]
[0161] Step 1: Synthesis of 1-(6-bromopyridin-2-yl)-3-ethoxycarbonyl-thiourea (compound 3).
[0162] At 5°C, ethoxycarbonyl isothiocyanate (6.80 mL, 57.8 mmol) was slowly added dropwise to 2-amino-6-bromopyridine (10.0 g, 57.8 mmol) in dichloromethane (100 mL) within 15 minutes. ) solution, after the dropwise addition, the reaction solution was warmed up to room temperature, and stirred overnight. The solvent was removed under reduced pressure, the solid was filtered, washed with petroleum ether, and dried in vacuo to obtain 16.9 g of yellow solid, yield: 96.1%.
[0163] Step 2: Synthesis of 5-bromo-[1,2,4]triazolo[1,5-a]pyridin-2-amine (Compound 4).
[0164] DIPEA (27.0mL, 165.6mmol) was added dropwise to a solution of hydroxylamine hydrochloride (19.2g, 276.0mmol) in ethanol / methanol (v:v=1:1, 170mL) a...
Embodiment 2
[0177] Example 2 N-(5-(4-((1,1-dioxo-4-thiomorpholinyl)methyl-d)phenyl-[1,2,4]triazolo [1,5-a]pyridin-2-yl)cyclopropanyl formamide (compound 18)
[0178]
[0179] Step 1: N-(5-(4-formylphenyl)-[1,2,4]triazolo[1,5-a]pyridin-2-yl)cyclopropanylcarboxamide (compound 15) synthesis.
[0180] N 2 Under protection, 1,4-dioxane (12mL) and water (4mL) were injected into N-(5-bromo-[1,2,4]triazolo[1,5-a]pyridine-2- base) cyclopropanyl formamide (560mg, 2.00mmol), (4-formylphenyl) boronic acid (360mg, 2.40mmol), Pd(dppf)Cl 2 (70mg, 0.10mmol), potassium carbonate (850mg, 6.00mmol) mixture, the reaction was carried out at 90°C overnight (16h). Cool to the greenhouse, filter with diatoms, wash the filter cake with dichloromethane, dry the filtrate with anhydrous sodium sulfate, remove the solvent, and carry out column separation of the concentrated solution (eluent: dichloromethane / methanol (v / v)=25: 1), to obtain 450 mg beige solid, yield: 73.5%. LC-MS(APCI):m / z=307.1(M+H) + . ...
Embodiment 3
[0187] Example 3 N-(5-(4-((1,1-dioxo-4-thiomorpholine)methyl-d2)phenyl)-[1,2,4]triazolo [1,5-a]pyridin-2-yl)cyclopropanylformamide (Compound 24)
[0188]
[0189] Step 1: Synthesis of 4-carboxymethyl phenylboronic acid (compound 20).
[0190] Concentrated sulfuric acid (0.5 mL) was added dropwise to 4-carboxyphenylboronic acid (3.50 g, 21.09 mmol) in anhydrous methanol (50 mL), and the mixture was refluxed overnight under nitrogen protection. The reaction solution was concentrated under reduced pressure, 50 mL of water was added, extracted with ethyl acetate (50x 3), the organic layer was washed with water (50 mL) and saturated brine (50 mL), dried over anhydrous sodium sulfate, and concentrated under reduced pressure to obtain a white solid 4.1 g, yield: 100%. LC-MS(APCI):m / z=181.1(M+H) + ; 1 H NMR (300MHz, DMSO-d 6 ): δ7.89(s,4H),7.52(s,4H),3.83(s,3H).
[0191] Step 2: Synthesis of methyl 4-(2-(cyclopropanylcarboxamido)-[1,2,4]triazolo[1,5-a]pyridin-5-yl)benzoate...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com