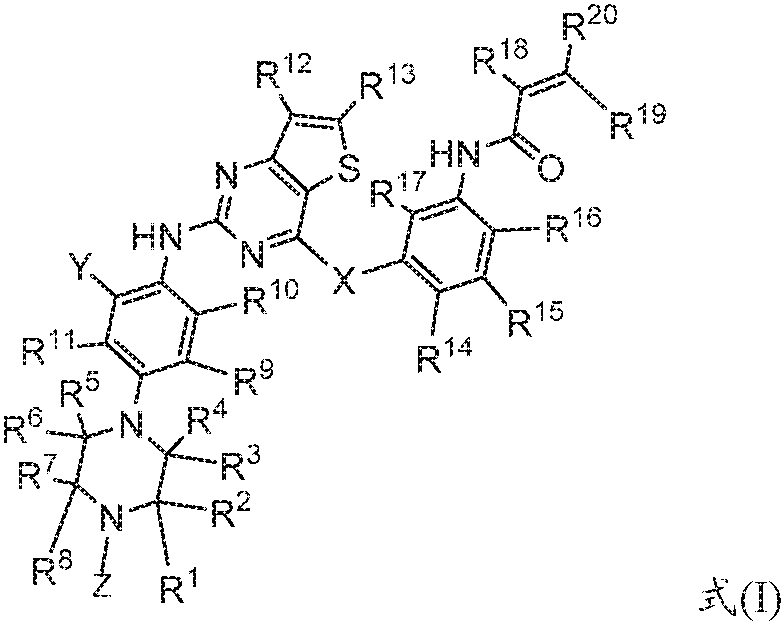
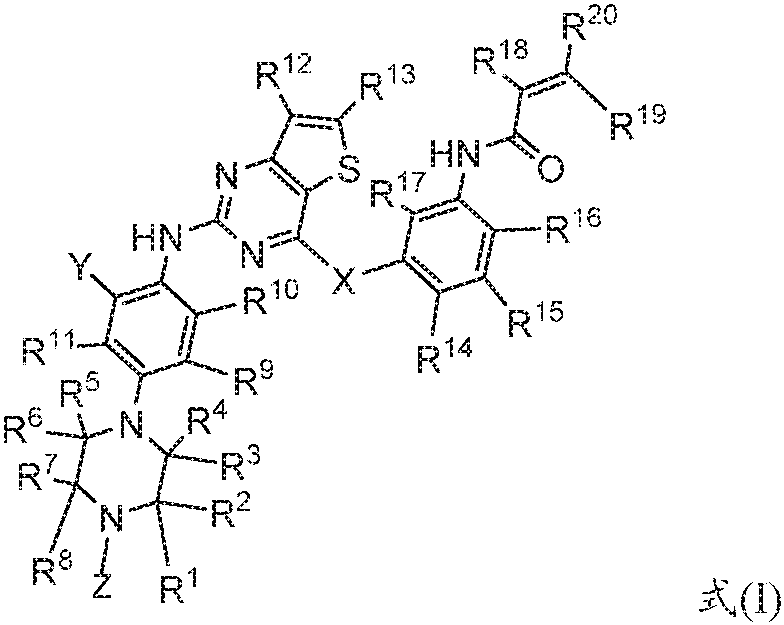
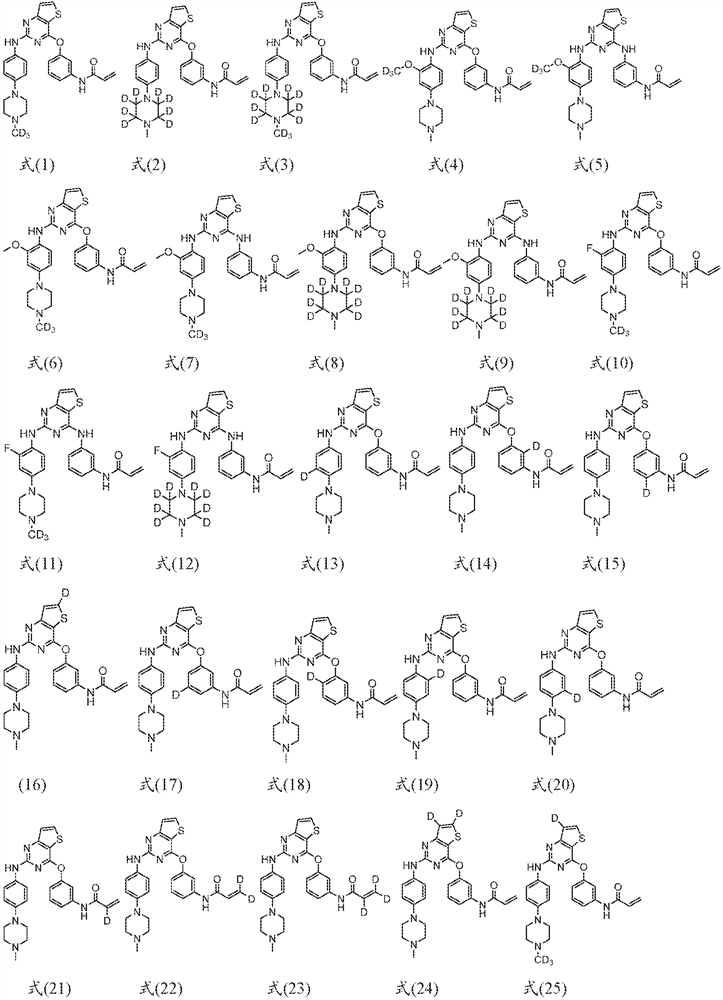
A kind of fused pyrimidine compound and the composition comprising the compound and its application
A technology for synthesizing pyrimidines and compounds is applied in the field of fused pyrimidine compounds and compositions containing the compounds, and can solve the problems of primary drug resistance or secondary drug resistance and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0138] The following synthetic route is used to prepare intermediate A-1: N-(3-(2-chlorothieno[3,2-d]pyrimidin-4-yloxy)phenyl)acrylamide, comprising the following steps:
[0139]
[0140] step one:
[0141] Under nitrogen protection, 2,4-dichlorothieno[3,2-d]pyrimidine (410mg, 2mmol), DMF (10mL), Cs 2 CO 3 (0.98g, 3mmol) and tert-butyl (3-hydroxyphenyl)carbamate (419mg, 2mmol) were successively added into a 50mL single-necked flask, stirred at room temperature for 2 hours, added 50mL of water, extracted with ethyl acetate, The organic phase was collected and purified by column chromatography to obtain a white solid product tert-butyl 3-(2-chlorothieno[3,2-d]pyrimidin-4-yloxy)phenylcarbamate (700mg, yield 92.6%) . 1 H NMR (300MHz, CDCl 3 )(δ / ppm) 8.01(d, J=5.4Hz.1H), 7.55(br s, 1H), 7.50(d, J=5.4Hz, 1H), 7.35(t, J=8.1Hz, 1H), 7.15-7.11(m, 1H), 6.97-6.94(m, 1H), 6.73(br, 1H), 1.51(s, 9H); LC-MS(APCI): m / z=377(M+1) + .
[0142] Step two:
[0143] Under nitrogen prote...
Embodiment 2
[0145] The following synthetic route is used to prepare intermediate B-1: N-(3-(2-chlorothieno[3,2-d]pyrimidin-4-ylamino)phenyl)acrylamide, comprising the following steps:
[0146]
[0147] step one:
[0148] Under nitrogen protection, 2,4-dichlorothieno[3,2-d]pyrimidine (410mg, 2mmol), n-butanol (n-BuOH, 10mL), N,N-diisopropylethylamine, (DIPEA, 390mg, 3mmol) and tert-butyl 3-aminophenylcarbamate (417mg, 2mmol) were added to a 50mL single-necked flask, stirred at room temperature for 16 hours, cooled to room temperature, and filtered to obtain a white solid Product (430 mg, yield 57%). 1 H NMR (300MHz, acetone-d6) (δ, ppm) 9.31 (br, 1H), 8.59 (br, 1H), 8.18-8.15 (m, 1H), 7.93-7.91 (m, 1H), 7.63-7.58 ( m, 1H), 7.38-7.32(m, 3H), 1.50(s, 9H); LC-MS(APCI): m / z=376.1(M+1) + .
[0149] Step two:
[0150] Under nitrogen, a solution of tert-butyl 3-(2-chlorothieno[3,2-d]pyrimidin-4-ylamino)phenylcarbamate (520 mg, 1.38 mmol) in dichloromethane (10 mL) was cooled To 0°C, trif...
Embodiment 3
[0153] The following synthetic route was used to prepare N-(3-(2-(2-d3-methoxy-4-(4-methylpiperazin-1-yl)phenylamino)thieno[3,2-d]pyrimidine -4-base oxy group) phenyl) acrylamide (formula (4)), comprises the following steps:
[0154]
[0155] step one:
[0156] Under nitrogen protection, 5-fluoro-2-nitrophenol (1.89g, 12mmol), acetonitrile (20mL), Cs 2 CO 3 (7.8g, 24mmol) and d3-methyl p-toluenesulfonate (CD 3 OTs, 3.42g, 18mmol) was added to a single-necked flask, refluxed for 3 hours, cooled to room temperature, added 50mL of water, extracted with dichloromethane, collected the organic phase, spin-dried and passed through the column to obtain the white solid product 4-fluoro-2-d3 - Methoxynitrobenzene (1.41 g, yield 67.3%). LC-MS(APCI): m / z=175.1(M+1) + .
[0157] Step two:
[0158] Under nitrogen protection, 4-fluoro-2-d3-methoxynitrobenzene (1.4g, 8mmol), DMF (15mL), K 2 CO 3 (2.2g, 16mmol) and N-methylpiperazine (1.2g, 12mmol) were added to a single-necked fla...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com