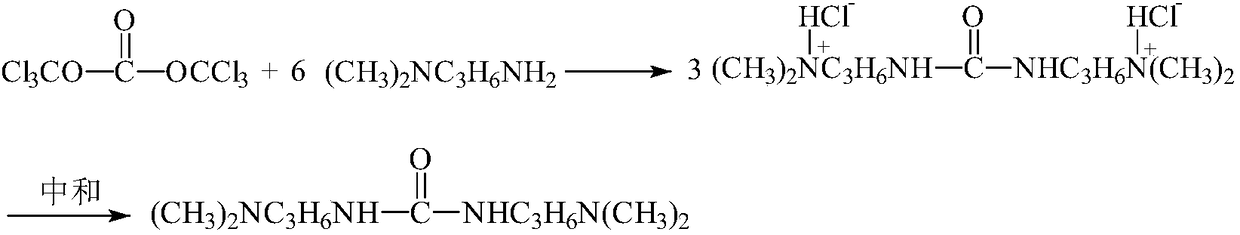
Synthetic process for N,N'-bi(3-dimethylaminopropyl)urea
A technology of dimethylaminopropyl and synthesis process, applied in the N field, can solve the problems of expensive ethylene carbonate, harsh operating conditions, high raw material cost, and achieve the effects of simple operation, less three wastes, and high product yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] In the present embodiment, the synthesis process of N, N'-bis(3-dimethylaminopropyl)urea is as follows:
[0029] Add 3 mol of N,N-dimethyl-1,3-propanediamine and solvent toluene to the reaction bottle, stir under cooling, control the temperature of the reaction solution below 10°C, add bis(trichloromethyl ) Carbonate 0.5mol toluene solution, drop it for 1 hour, continue to stir and react for 2 hours after dropping; then add 3mol saturated aqueous solution of potassium carbonate to neutralize, install the water separator, raise the temperature and separate the water, observe the water separator until no water comes out Cooled to room temperature, filtered, the filtrate was distilled off toluene under reduced pressure to obtain the target product N, N'-bis(3-dimethylaminopropyl)urea, the yield was 91%, and the gas chromatography analysis purity was greater than 98%. IR (cm -1 ):761,1038,1267,1636,1941,3325 have absorption peaks;
[0030] 1 H-NMR (D 2 O, ppm): 1.45-1.5...
Embodiment 2
[0032] In the present embodiment, the synthesis process of N, N'-bis(3-dimethylaminopropyl)urea is as follows:
[0033] Add 3 mol of N,N-dimethyl-1,3-propanediamine and solvent benzene into the reaction bottle, stir under cooling, control the temperature of the reaction solution below 10°C, add bis(trichloromethyl ) Carbonate 0.5mol benzene solution, drop it for 1h, continue to stir and react for 2h after dripping; then add 3mol saturated aqueous solution of potassium carbonate to neutralize, install the water separator, raise the temperature and divide the water, observe the water separator until no water comes out Cooled to room temperature, filtered, and the filtrate was distilled off under reduced pressure to remove benzene to obtain the target product N, N'-bis(3-dimethylaminopropyl) urea with a yield of 92% and a gas chromatographic analysis purity greater than 98%.
Embodiment 3
[0035] In the present embodiment, the synthesis process of N, N'-bis(3-dimethylaminopropyl)urea is as follows:
[0036] Add 3 mol of N,N-dimethyl-1,3-propanediamine and solvent toluene to the reaction bottle, stir under cooling, control the temperature of the reaction solution below 10°C, add bis(trichloromethyl ) Carbonate 0.5mol toluene solution, drop it for 1 hour, continue to stir for 2 hours after dropping; then add 3mol saturated aqueous solution of sodium carbonate to neutralize, install the water separator, raise the temperature and separate the water, observe the water separator until no water comes out Cooled to room temperature, filtered, the filtrate was distilled off toluene under reduced pressure to obtain the target product N,N'-bis(3-dimethylaminopropyl)urea, the yield was 90%, and the gas chromatography analysis purity was greater than 98%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com