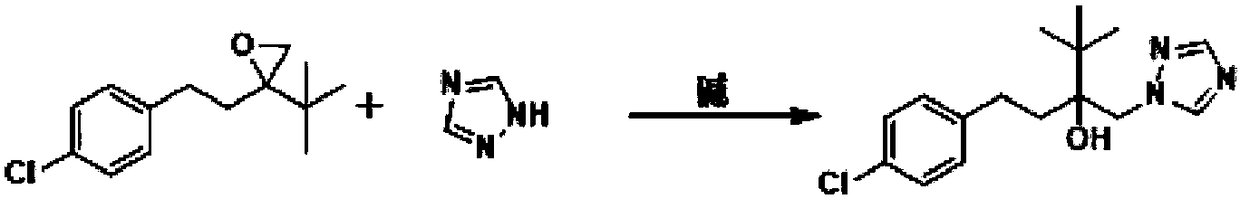
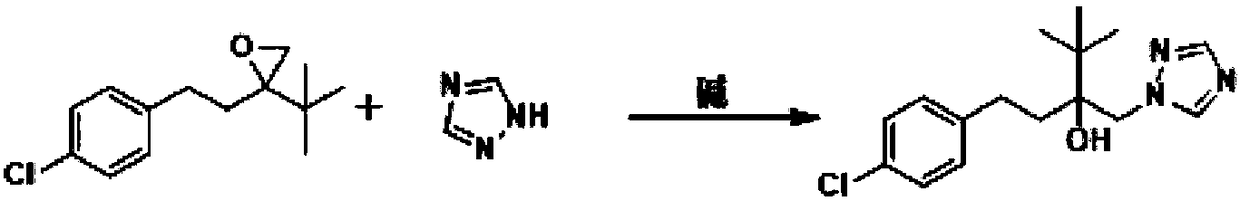
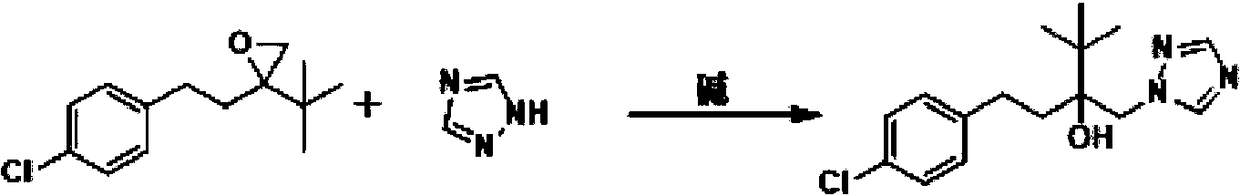
Simple new process method for synthesizing tebuconazole in water phase
A technology of tebuconazole and a new method, applied in the field of water-phase synthesis of tebuconazole, can solve the problems of difficult solvent recovery, long reaction time, loss of product yield and the like, and achieves simple synthesis and post-processing purification process, and the reaction time is convenient. The effect of shortening and high product yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Add 23.8 g of tebuconazole epoxy compound, 120 mL of water, 7.9 g of triazole, 25.2 g of potassium hydroxide, and 0.2 g of tetrabutylammonium chloride into a 500 ml three-necked flask. Warm up to reflux and react for 4h. The normalized content of 4H isomer was determined to be 0.7% by sampling. After cooling down to 10-15° C. and stirring for 1 hour, a large amount of white solid was precipitated, and 30.5 g of the product was obtained after suction filtration and drying, with a yield of 97.4% and a content of 98.1%. The filtrate continued to be applied mechanically to the next batch of synthesis reactions.
Embodiment 2
[0024] Add above-mentioned suction filtration filtrate 143g in the 500ml there-necked flask, then add tebuconazole epoxy compound 23.8g, triazole 7.9g, potassium hydroxide 2.5g. Warm up to reflux and react for 4h. The normalized content of 4H isomer was determined to be 0.5% by sampling. After cooling down to 10-15° C. and stirring for 1 h, a large amount of white solid was precipitated, and 30.4 g of the product was obtained after suction filtration and drying, with a yield of 97.2% and a content of 98.0%. The filtrate can be applied to more than 10 batches.
Embodiment 3
[0026] Add 23.8g of tebuconazole epoxy compound, 160mL of water, 9.4g of triazole, 22.4g of potassium hydroxide, and 4000.5g of polyethylene glycol into a 500ml three-necked flask. Warm up to reflux and react for 3h. The normalized content of 4H isomer was determined to be 1.0% by sampling. After cooling down to 10-15° C. and stirring for 1 hour, a large amount of white solid was precipitated, and 29.7 g of the product was obtained after suction filtration and drying, with a yield of 94.8% and a content of 97.8%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com