Medical hemostatic material capable of rapidly forming film after being sprayed, and application method of medical hemostatic material
A hemostatic material, a technology of spray film formation, applied in the field of medical materials, can solve the problems of difficult operation, difficult to effectively stop bleeding, secondary damage, etc., to achieve good biocompatibility, reduce wound damage, and promote healing effects.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
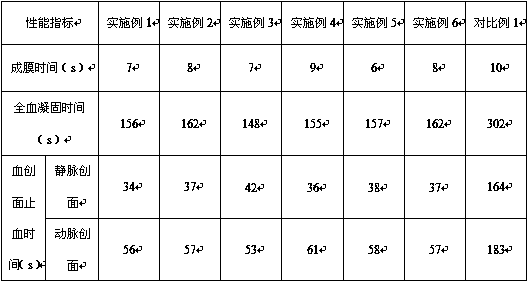
Examples
Embodiment 1
[0030] The hemostatic material consists of:
[0031] The medical hemostatic material consists of liquid A and liquid B.
[0032] The preparation process of liquid A is: add 10 parts by weight of silica airgel powder, 13 parts by weight of chitosan microspheres, 7 parts by weight of graphene and 13 parts by weight of gelatin particles into 57 parts by weight of natural rubber emulsion, ultrasonically disperse Uniform, prepared A liquid; the average particle size of silica airgel powder is 6μm, and the density is 0.09g / cm 3 , the porosity is 95%; the average particle size of chitosan microspheres is 12μm, and the porosity is 94%; graphene is single-layer graphene, the average particle size is 14μm, and the specific surface area is 2200m 2 / g, the porosity is 95%; the gelatin particles are skin gelatin particles, the average particle size is 32 μm; the solid content of the natural rubber emulsion is 46%, the pH value is 6.5, and the rubber hydrocarbon content in the natural rubb...
Embodiment 2
[0041] The hemostatic material consists of:
[0042] The medical hemostatic material consists of liquid A and liquid B.
[0043] The preparation process of liquid A is: add 8 parts by weight of silica airgel powder, 10 parts by weight of chitosan microspheres, 6 parts by weight of graphene and 11 parts by weight of gelatin particles into 65 parts by weight of natural rubber emulsion, and ultrasonically disperse Uniform, prepared A liquid; the average particle size of silica airgel powder is 4μm, and the density is 0.06g / cm 3 , the porosity is 95%; the average particle size of chitosan microspheres is 5 μm, and the porosity is 92%; graphene is double-layer graphene, the average particle size is 10 μm, and the specific surface area is 2000 m 2 / g, the porosity is 94%; the gelatin particles are bone gelatin particles, the average particle size is 20 μm; the solid content of the natural rubber emulsion is 40%, the pH value is 6, and the rubber hydrocarbon content in the natural r...
Embodiment 3
[0052] The hemostatic material consists of:
[0053] The medical hemostatic material consists of liquid A and liquid B.
[0054] The preparation process of liquid A is: add 12 parts by weight of silica airgel powder, 15 parts by weight of chitosan microspheres, 10 parts by weight of graphene and 14 parts by weight of gelatin particles into 49 parts by weight of natural rubber emulsion, ultrasonically disperse Uniform, prepared A liquid; the average particle size of silica airgel powder is 10 μm, and the density is 0.12g / cm 3 , the porosity is 998%; the average particle size of chitosan microspheres is 20μm, and the porosity is 96%; graphene is few-layer graphene, the average particle size is 20μm, and the specific surface area is 2500m 2 / g, the porosity is 99%; the gelatin particles are tendon gelatin particles, the average particle size is 40 μm; the solid content of natural rubber emulsion is 50%, the pH value is 7, and the content of rubber hydrocarbon in natural rubber i...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| density | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com