Degradation method of organic pollutants
A technology of organic pollutants and nickel hydroxide, applied in water pollutants, chemical instruments and methods, water/sewage treatment, etc., can solve problems such as high toxicity, limited popularization and application, and no activation of PS, and achieve stable properties, High degradation efficiency, beneficial to industrial application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] 1. Preparation of nickel hydroxide: Ni(NO 3 ) 2 ·6H 2 O and urea were dissolved in a mixed solution of water and ethylene glycol, stirred and mixed evenly, then transferred to a hydrothermal kettle, placed in an oven at 120°C for 4 hours, and after the reaction kettle was cooled to room temperature, the obtained product was watered Alternately washing with ethanol and centrifuging for three times, finally vacuum drying and grinding to obtain nickel hydroxide.
[0041] Ni(NO 3 ) 2 ·6H 2 The molar ratio of O to urea is 1:4.
[0042] The volume ratio of water and ethylene glycol described in step 1 is 1:1.
[0043] Ni(NO 3 ) 2 ·6H 2 The concentration of O in the mixed solution of water and ethylene glycol is 0.06mol / L and the concentration of urea in the mixed solution of water and ethylene glycol is 0.25mol / L.
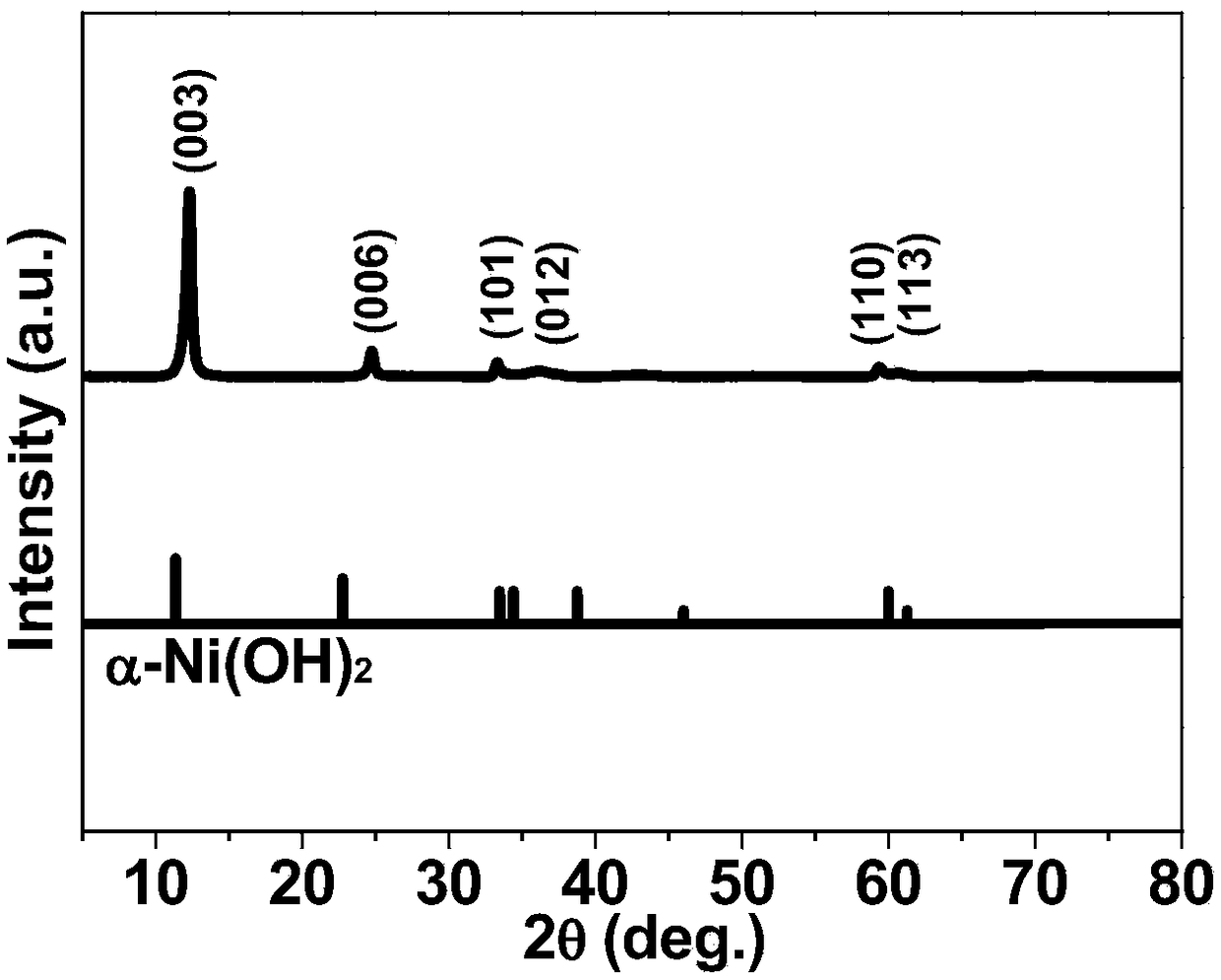
[0044] Nickel hydroxide as described in step 1, such as figure 1 As shown, its XRD peak completely corresponds to the Jade spectrum of standard α-phase...
Embodiment 2
[0057] 1. Preparation of nickel hydroxide: Ni(NO 3 ) 2 ·6H 2 O and urea were dissolved in a mixed solution of water and ethylene glycol, stirred and mixed evenly, then transferred to a hydrothermal kettle, placed in an oven at 120°C for 4 hours, and after the reaction kettle was cooled to room temperature, the obtained product was watered Alternately washing with ethanol and centrifuging for three times, finally vacuum drying and grinding to obtain nickel hydroxide.
[0058] Ni(NO 3 ) 2 ·6H 2 The molar ratio of O to urea is 1:3.
[0059] The volume ratio of water and ethylene glycol described in step 1 is 1:0.5.
[0060] Ni(NO 3 ) 2 ·6H 2 The concentration of O in the mixed solution of water and ethylene glycol is 0.04mol / L and the concentration of urea in the mixed solution of water and ethylene glycol is 0.20mol / L.
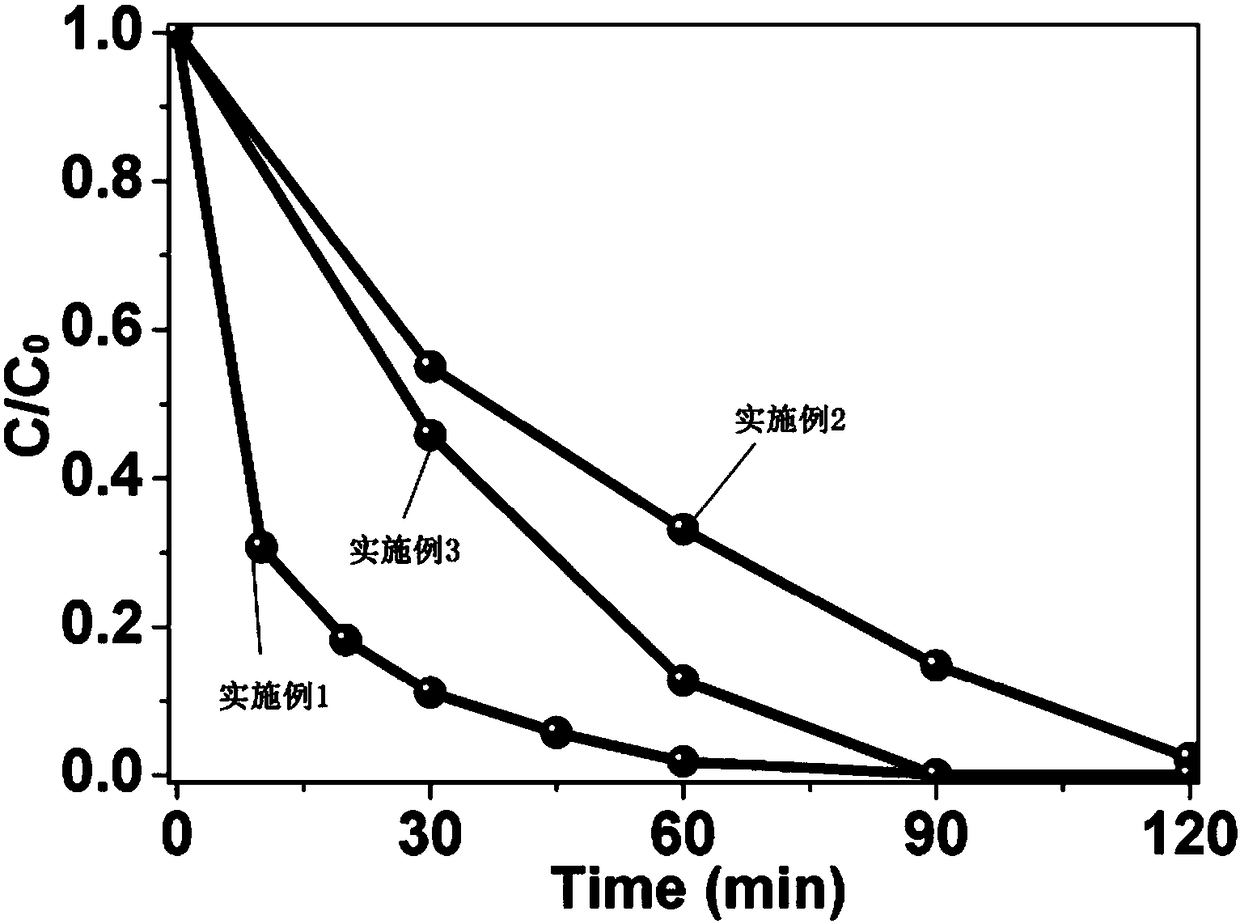
[0061] 2. Put the prepared nickel hydroxide into a solution containing refractory organic pollutants such as phenol, and at the same time, put a certa...
Embodiment 3
[0072] 1. Preparation of nickel hydroxide: Ni(NO 3 ) 2 ·6H 2 O and urea were dissolved in a mixed solution of water and ethylene glycol, stirred and mixed evenly, then transferred to a hydrothermal kettle, placed in an oven at 120°C for 4 hours, and after the reaction kettle was cooled to room temperature, the obtained product was watered Alternately washing with ethanol and centrifuging for three times, finally vacuum drying and grinding to obtain nickel hydroxide.
[0073] Ni(NO 3 ) 2 ·6H 2 The molar ratio of O to urea is 1:5.
[0074] The volume ratio of water and ethylene glycol described in step 1 is 1:1.5.
[0075] Ni(NO 3 ) 2 ·6H 2 The concentration of O in the mixed solution of water and ethylene glycol is 0.08mol / L and the concentration of urea in the mixed solution of water and ethylene glycol is 0.30mol / L.
[0076] 2. Put the prepared nickel hydroxide into a solution containing refractory organic pollutants such as phenol, and at the same time, put a certa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com