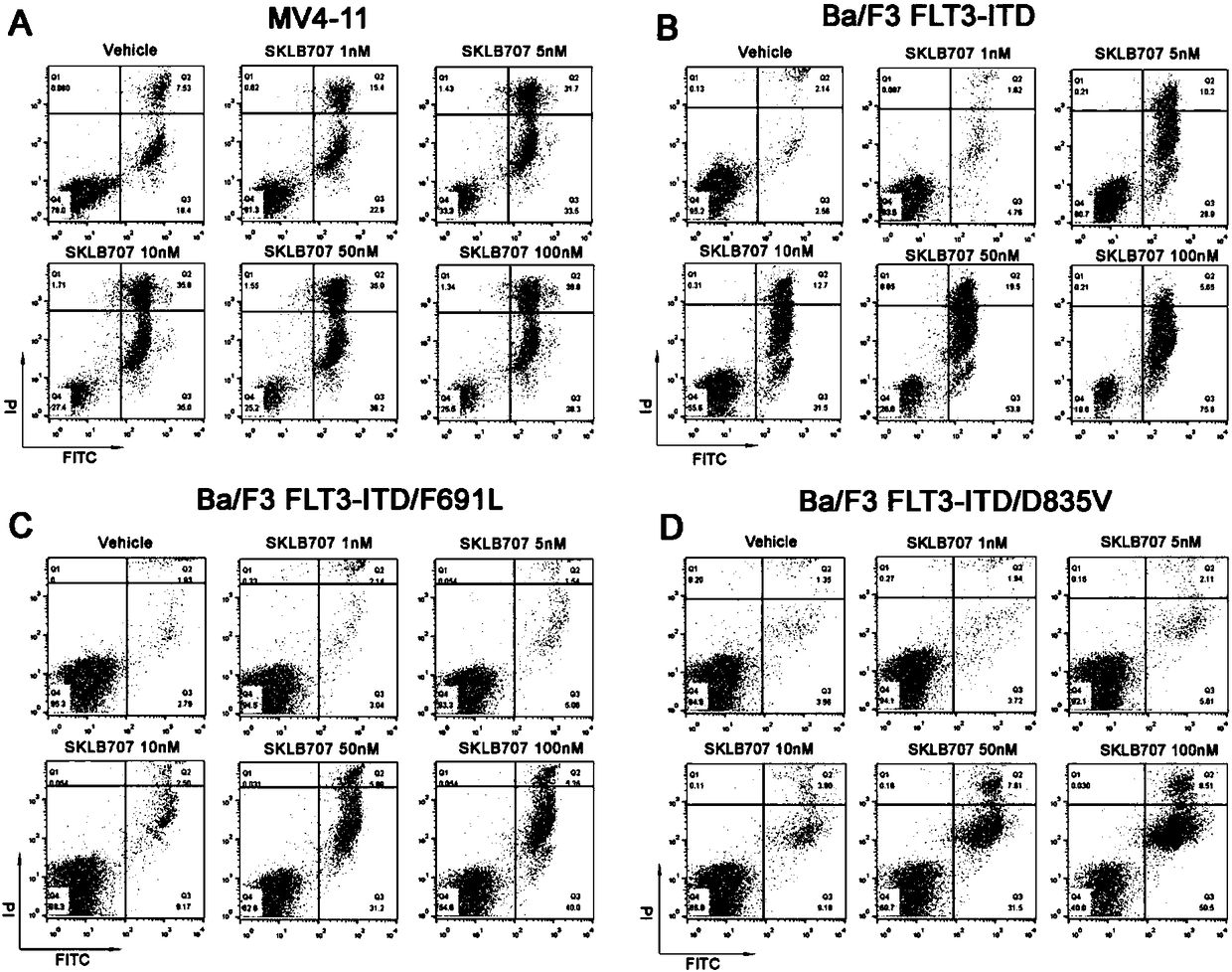
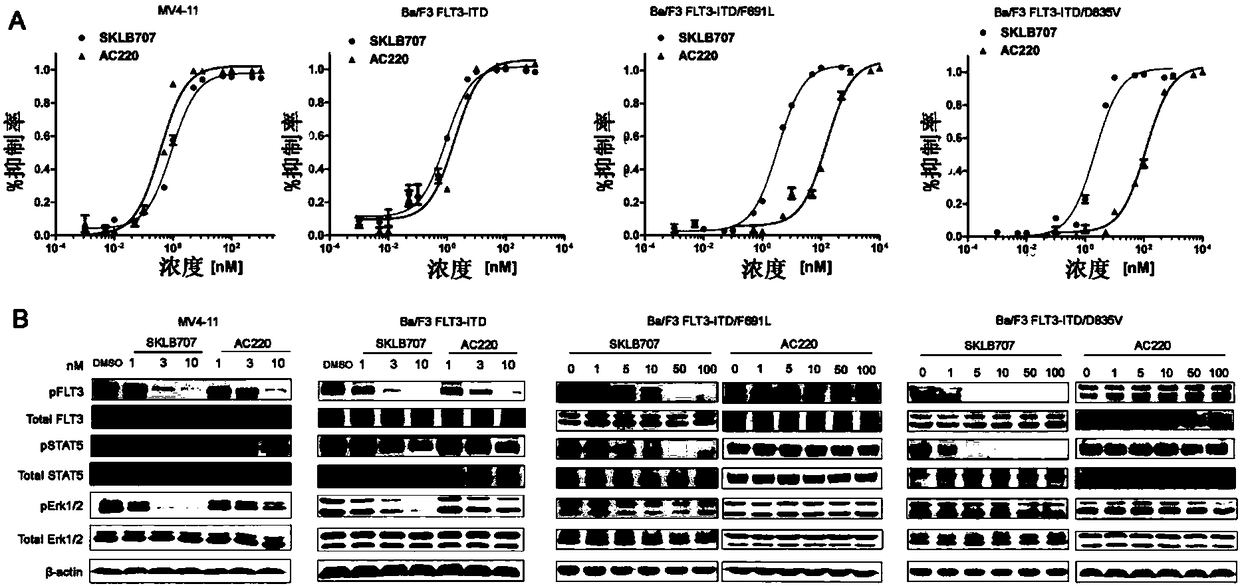
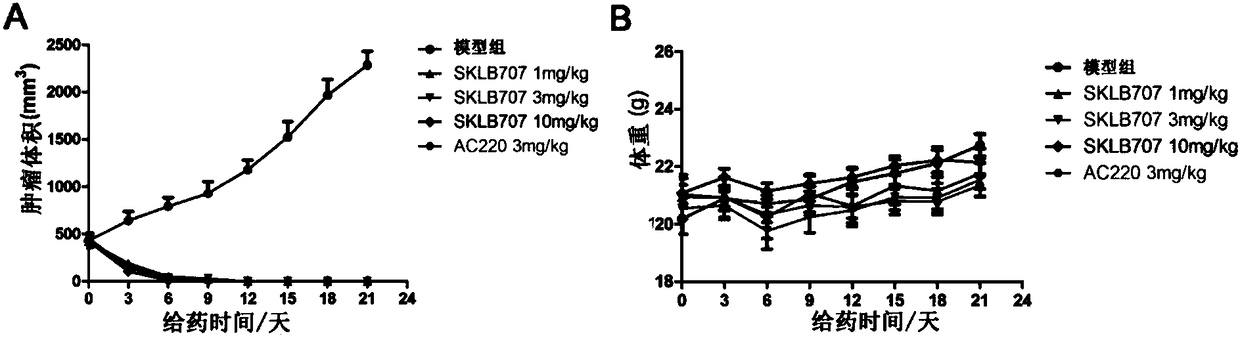
Substituted 1-(isoxazol-3-yl)-3-(3-fluoro-4-phenyl)urea derivative and preparation method and use thereof
A technology of urea derivatives and isoxazoles, applied in the field of organic synthetic drugs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0088] Example 1 Preparation of 3-amino-5-tert-butylisoxazole (intermediate 2)
[0089]
[0090] Weigh raw material 1 (4,4-dimethyl-3-oxopentonitrile) (1.25g, 10mM) in a 250mL round bottom flask filled with 100mL of water, then add hydroxylamine hydrochloride (759mg, 11mM) and hydroxide Sodium (440mg, 11mM), the reaction solution was stirred at room temperature for half an hour, then an appropriate amount of sodium hydroxide was added to adjust the pH to 8-9, and then the temperature was raised to 50°C for 10h. After the TCL detection reaction was complete, 100 mL of methyl tert-butyl ether was added into the solution three times for extraction, the organic layer was discarded, and 150 mL of DCM (dichloromethane) was added to the water layer for three times for extraction, and the DCM layer was collected. Wash with saturated brine three times, then add anhydrous sodium sulfate to dry, filter and spin the filtrate to give white solid N'-hydroxy-4,4-dimethyl-3-carbonylpentami...
Embodiment 2
[0094] Example 2 Preparation of 1-(5-(tert-butyl)isoxazol-3-yl)-3-(3-fluoro-4-hydroxyphenyl)urea (Intermediate 3)
[0095]
[0096] Weigh triphosgene (5.282g, 17.8mM) in a 500mL round bottom flask, then add 200mL of ethyl acetate, then slowly add 100mL of intermediate 2 (5g, 35.7mM) ethyl acetate dropwise to the flask in an ice-water bath environment After the ester solution was added dropwise, triethylamine (7.2 g, 71.4 mM) was slowly added dropwise in an ice-water bath environment, and then the temperature was raised to 70° C. to react for three hours. After the reaction was completed, the organic phase was removed directly with a rotary evaporator, and then 200 mL of ethyl acetate solution of 4-amino-2-fluorophenol (4.4 g, 35 mM) was added, and the reaction solution was heated at 70 °C for 5 h, and detected by TCL. After the reaction is complete, remove the organic phase in the reaction liquid with a rotary evaporator, add 200 mL of a saturated aqueous solution of potass...
Embodiment 3
[0098] Preparation of Example 3 tert-butyl (6-fluoropyridin-3-yl) carbamate (intermediate 5)
[0099]
[0100]In a 250mL round-bottomed flask, the intermediate 4(6-fluoropyridin-3-amine) (2g, 15.6mM) and BOC anhydride (di-tert-butyl dicarbonate, 4g, 18.7mM) were added, followed by 150mL of dioxane The ring was used as a solvent, then the temperature was raised to 100°C, and the reaction was carried out for about 8 hours until the reaction was detected by TLC. Then the reaction solution was concentrated under reduced pressure, water and dilute hydrochloric acid were added to adjust the pH to 3-4, and then 150 mL of ethyl acetate was added for extraction, which was added to the water layer in three times, and the ethyl acetate layer was collected. Then the ethyl acetate layer was washed three times with saturated brine, then the ethyl acetate layer was dried with anhydrous sodium sulfate, and after filtering, the filtrate was directly removed with a rotary evaporator to obtai...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com