Method for producing polyethylene glycol having terminal carboxy group
A polyethylene glycol and production method technology, applied in the production field of polyethylene glycol with terminal carboxyl groups, to achieve the effect of inhibiting the increase of polydispersity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example
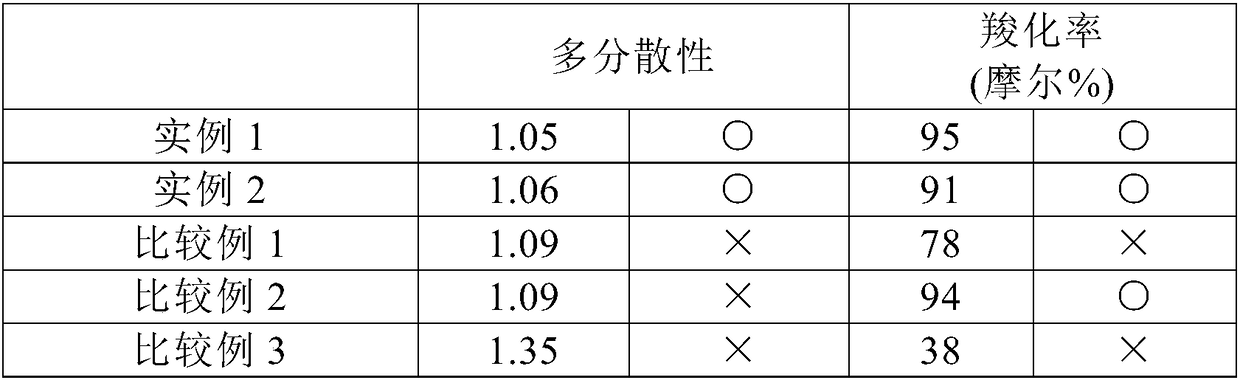
[0057] The present invention will be described more specifically with reference to examples. In addition, "%" hereinafter means "% by weight" unless otherwise specified.
[0058] The polydispersity and carboxylation rate of the polyethylene glycols obtained in Examples 1 to 2 and Comparative Examples 1 to 3 were calculated by using liquid chromatography (GPC and HPLC). As a system for liquid chromatography, Alliance manufactured by Waters Corp. was used.
[0059] The analysis conditions of GPC and HPLC are as follows.
[0060] GPC analysis
[0061] Detector: Differential refractometer
[0062] Columns: Ultrahydrogel 500 and Ultrahydrogel 250 (Waters Corp.)
[0063] Mobile phase: sodium acetate buffer (solvent: water, pH: 5.2)
[0064] Flow rate: 0.5ml / min
[0065] Sample volume: 5mg / mL, 20μL
[0066] Column temperature: 30°C
[0067] HPLC analysis
[0068] Detector: Differential refractometer
[0069] Column: Anion exchange column ES-502N (Asahipak)
[0070] Mobile ...
example 1
[0075] Sodium bicarbonate (0.27 g) was dissolved in ion-exchanged water (4.0 g) to obtain an aqueous sodium bicarbonate solution. Next, stir the double salt of potassium peroxymonosulfate, potassium bisulfate and potassium sulfate (0.25 g, content of potassium peroxymonosulfate=45% in the double salt, with respect to 1 mole of The amount of potassium persulfate of methoxypolyethylene glycol = 1.8 moles, produced by Tokyo Chemical Industry Co., Ltd.) and ethylenediaminetetraacetic acid (5.5 mg, 1.9 × 10 -5 Mole, manufactured by Kanto Chemical Co., Inc.). To the obtained suspension, an aqueous sodium bicarbonate solution was added dropwise to dissolve ethylenediaminetetraacetic acid, thereby obtaining an aqueous potassium peroxymonosulfate solution. Then, dissolve methoxypolyethylene glycol (2.0 g, 4.0×10 -4 Mole, weight average molecular weight = about 5,000, in formula (I), n = about 113, m = 2, SUNBRIGHT ME-050AL produced by NOF Corp.), obtained under a nitrogen atmosphere ...
example 2
[0080] Sodium bicarbonate (0.14 g) was dissolved in ion-exchanged water (2.0 g) to obtain an aqueous sodium bicarbonate solution. Next, stir the double salt (18 mg of potassium peroxymonosulfate, potassium bisulfate and potassium sulfate in ion-exchanged water (3.0 g), the content of potassium peroxymonosulfate=45% in the double salt, relative to 1 mole of Amount of potassium persulfate of methoxypolyethylene glycol = 1.6 mol, produced by Tokyo Chemical Industry Co., Ltd.) and ethylenediaminetetraacetic acid (0.4 mg, 1.3 x 10 -6 mole, produced by Kanto Chemical Co., Inc.). To the obtained suspension, an aqueous sodium bicarbonate solution was added dropwise to dissolve ethylenediaminetetraacetic acid, thereby obtaining an aqueous potassium peroxymonosulfate solution. Then, methoxypolyethylene glycol (1.0 g, 3.3×10 -5 Mole, weight average molecular weight=about 30,000, in formula (I), n=about 680, m=2, SUNBRIGHT ME-300AL produced by NOFCorp.), to the solution obtained under n...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com