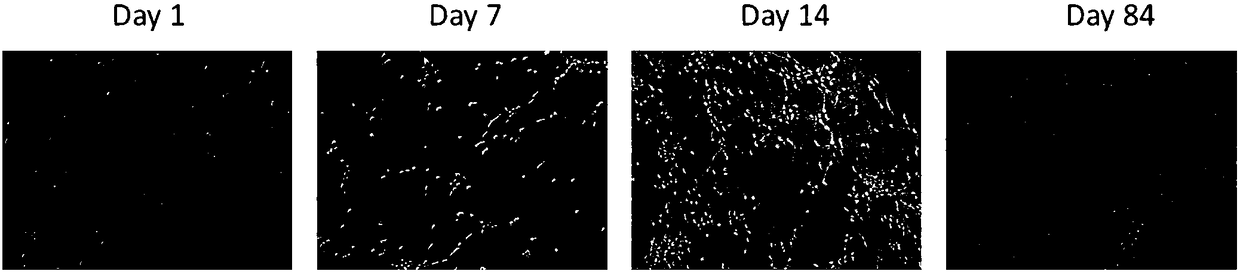
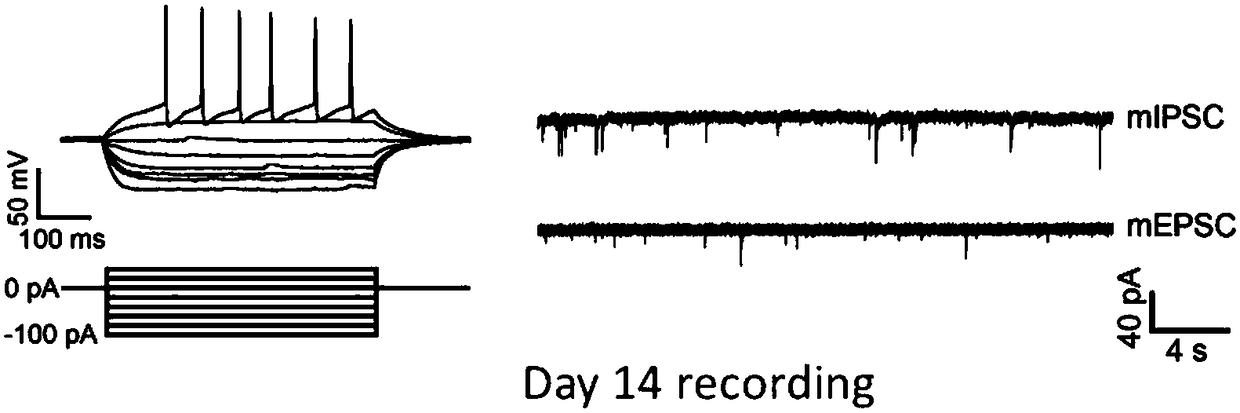
Induced differentiation method of functional cerebral cortical cells
A technique for inducing differentiation of the cerebral cortex, applied in the direction of biochemical equipment and methods, animal cells, nervous system cells, etc., can solve problems such as limitations, long culture time, unsuitable for re-changing liquids, etc., to shorten the production cycle and reduce manufacturing costs. cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Step 1. After digesting the neural precursor cells (hNPC) obtained by differentiation of the human induced pluripotent stem cell line DYR0100 with accutase, press 5×10 5 / cm 2 Inoculate onto poly-D-lysine and laminin-coated cell culture plates;
[0049] Step 2. Use Neural Differentiation Medium A from day 1 at 37°C, 5% CO 2 Cultured in the cell culture box for 7 days, half of the medium was changed every 3 days; the neural differentiation medium A contained final concentrations of 2 μM retinoic acid, 20ng / ml BDNF, 20ng / ml GDNF, 0.2mM ascorbic acid, 100nM SU5402, 200ng / ml The Neurobasal medium of ml BIBF1120, 10μM IBMX and 5mM glucose and the B27 supplement without vitamin A, wherein the dosage ratio of the Neurobasal medium and the B27 supplement is 50:1;
[0050] Step 3. Use Neural Differentiation Medium B from day 7 at 37°C, 5% CO 2 Culture in a cell culture incubator, half of the medium was changed every 3 days; neural differentiation medium B contained final conc...
Embodiment 2
[0065] Step 1. After digesting the neural precursor cells (hNPC) obtained by differentiation of the human induced pluripotent stem cell line DYR0100 with accutase, press 5×10 5 / cm 2 Inoculate onto poly-D-lysine and laminin-coated cell culture plates;
[0066] Step 2. Use Neural Differentiation Medium A from day 1 at 37°C, 5% CO 2 Cultivate in the cell culture box for 7 days, and change the medium in half every 3 days; the neural differentiation medium A contains Neurobasal culture with a final concentration of 2μM retinoic acid, 20ng / ml BDNF, 20ng / ml GDNF, 0.2mM ascorbic acid, and 10μM SU5402 Base and B27 supplement without vitaminA, wherein the dosage ratio of Neurobasal medium and B27 supplement is 50:1;
[0067] Step 3. Use Neural Differentiation Medium B from day 7 at 37°C, 5% CO 2 Culture in the cell culture incubator and change the medium every 3 days; neural differentiation medium B contains neurobasal medium with a final concentration of 20ng / ml BDNF, 20ng / ml GDNF,...
Embodiment 3
[0073] Step 1. After digesting the neural precursor cells (hNPC) obtained by differentiation of the human induced pluripotent stem cell line DYR0100 with accutase, press 5×10 5 / cm 2 Inoculate onto poly-D-lysine and laminin-coated cell culture plates;
[0074] Step 2. Use Neural Differentiation Medium A from day 1 at 37°C, 5% CO 2 Cultured in the cell culture box for 7 days, half of the medium was changed every 3 days; the neural differentiation medium A contained final concentrations of 2 μM retinoic acid, 20 ng / ml BDNF, 20 ng / ml GDNF, 0.2 mM ascorbic acid, 5 μM SU5402, 50 μM IBMX and the Neurobasal medium of 10mM glucose and the B27 supplement without vitamin A, wherein the dosage ratio of the Neurobasal medium and the B27 supplement is 50:1;
[0075] Step 3. Use Neural Differentiation Medium B from day 7 at 37°C, 5% CO 2 Culture in a cell culture incubator, and change the medium every 3 days; neural differentiation medium B contains neurobasal medium with a final concent...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com