Method for realizing asymmetric reduction of p-propiophenone compounds by adopting carrot tissue or callus and applications of carrot tissue or callus in asymmetric reduction of p-propiophenone compounds
A carrot and propiophenone technology, applied in the biological field, can solve the problems of different catalytic activity of plant cells, high price of synthetic drugs, etc., and achieve the effects of great economic and ecological significance, simple and economical access, and popularization of reagents.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
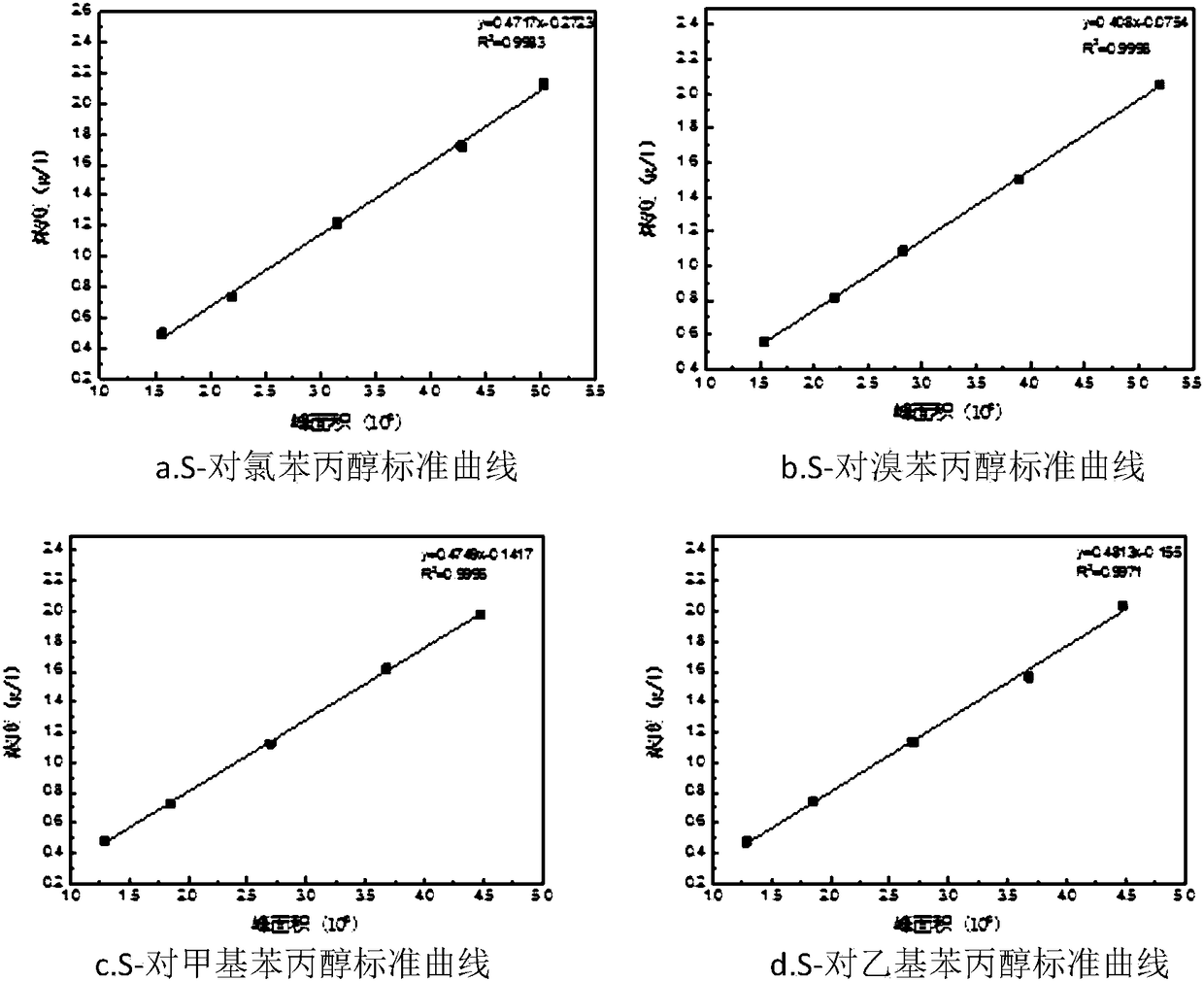
[0058] Embodiment 1: the preparation of standard substance.
[0059] Dissolve 5g of p-chloropropiophenone in 50ml of methanol and add KBH several times 4 The one-pot method was used to react in an ice-water bath, using petroleum ether: ethyl acetate = 9:1 as the developing agent to spot the plate, until the reaction was complete, add an appropriate amount of ice water to remove excess KBH 4 , and then adjust the pH value to neutral with dilute HCl, extract the product with anhydrous ether about 20ml each time, and extract three times. After adding anhydrous MgSO 4After drying, filtration and rotary evaporation, the standard p-chlorophenylpropanol was obtained.
[0060] For p-bromopropiophenone, p-methylpropiophenone and p-ethylpropiophenone, the same method can be used to obtain corresponding standard substances.
[0061] The standard curves for the four substrates are as follows figure 1 shown.
Embodiment 2
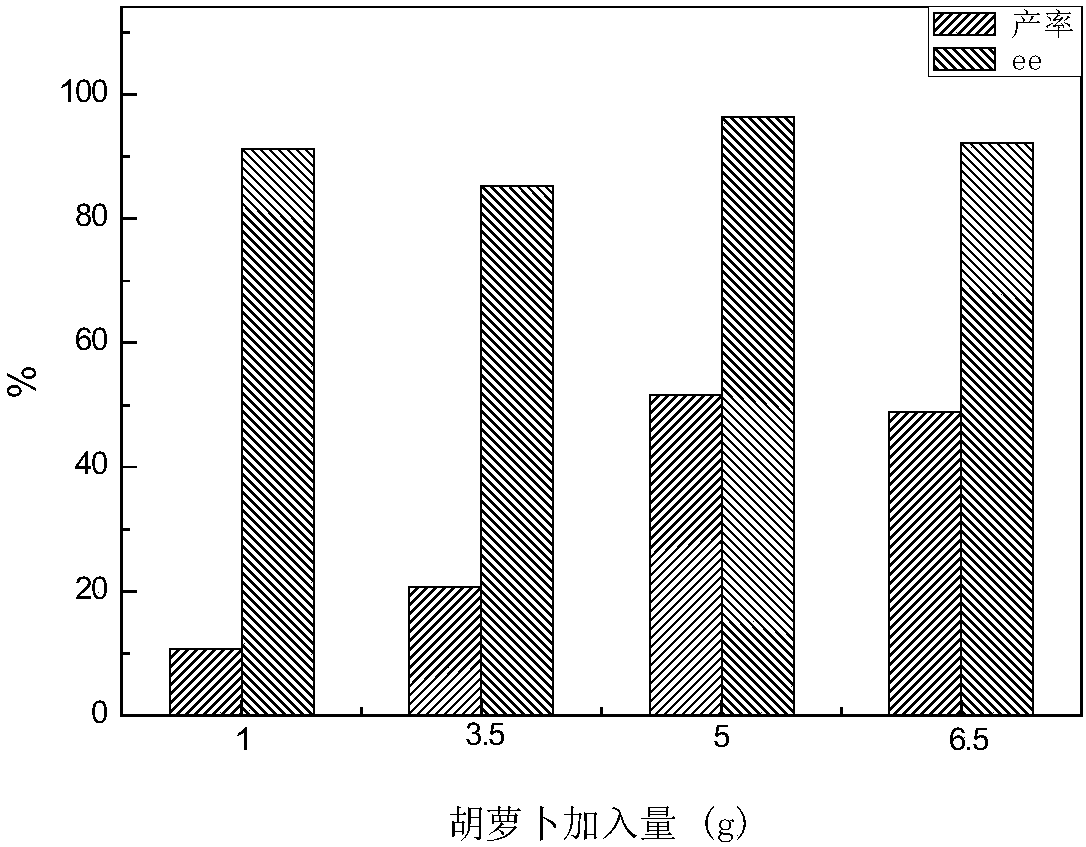
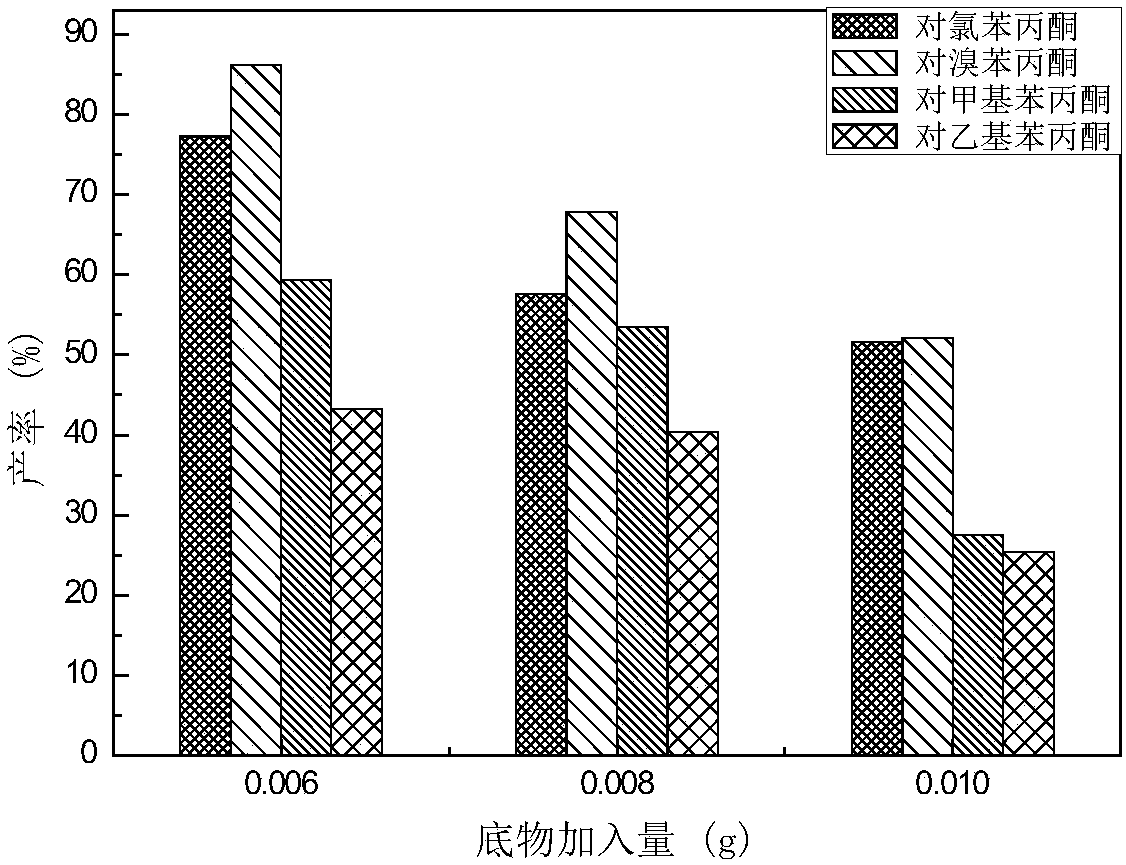
[0062] Example 2: Consideration of asymmetric reduction of substrates by different plant tissues.
[0063] Rinse different kinds of plants with clean water to remove surface stains, soak them in 75% ethanol for 0.5min, take them out, rinse them twice with sterile water, put them in 10% NaClO solution for 20mins, take them out, rinse them with sterile water several times , cut off the surface 0.3cm, cut into small pieces of the same species or leaves of the same weight for later use.
[0064] After the carrots and the different plants purchased were sterilized according to the above method, 5g (wet weight) was put into 20ml pH5.8 (adjust pH with NaOH or phosphoric acid) culture medium and 0.5g / l different bottom After reacting for 5 days with substances (p-methylpropiophenone, p-ethylpropiophenone, p-chloropropiophenone, p-bromopropiophenone), centrifuge, extract, rotary evaporate, constant volume, and detect by liquid chromatography.
[0065] The reducing ability of different...
Embodiment 3
[0070] Example 3: Consider the influence of different sampling sites on the yield and ee value.
[0071] Select the phloem and xylem of carrot respectively as biocatalyst, after the same method operation by embodiment 2, the result is as shown in table 3:
[0072] Table 3: Effects of different parts of carrots on the reaction
[0073]
[0074] It can be seen from the above table that the phloem of carrot has a strong reducing ability, but its xylem basically has no reducing ability; therefore, carrot phloem is selected as the biotransformation material in subsequent experiments.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com