High-cycle-stability lithium-sulfur electrolyte
A technology of cycle stability and electrolyte, applied in lithium batteries, organic electrolytes, non-aqueous electrolytes, etc., can solve the problem of not greatly improving the cycle stability of lithium-sulfur batteries, and achieve broad industrialization prospects, low cost, strong The effect of chemical adsorption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Put 0.1 mol of lithium trifluoromethanesulfonate in a glove box for sufficient drying, then add 100 mL of ethylene glycol dimethyl ether and 1,3 dioxolane mixed organic solvent with a volume ratio of 1:1 , fully stirred until completely dissolved, then added 0.5g of carbon quantum dots, stirred and dispersed evenly to obtain a brown-yellow lithium-sulfur electrolyte.
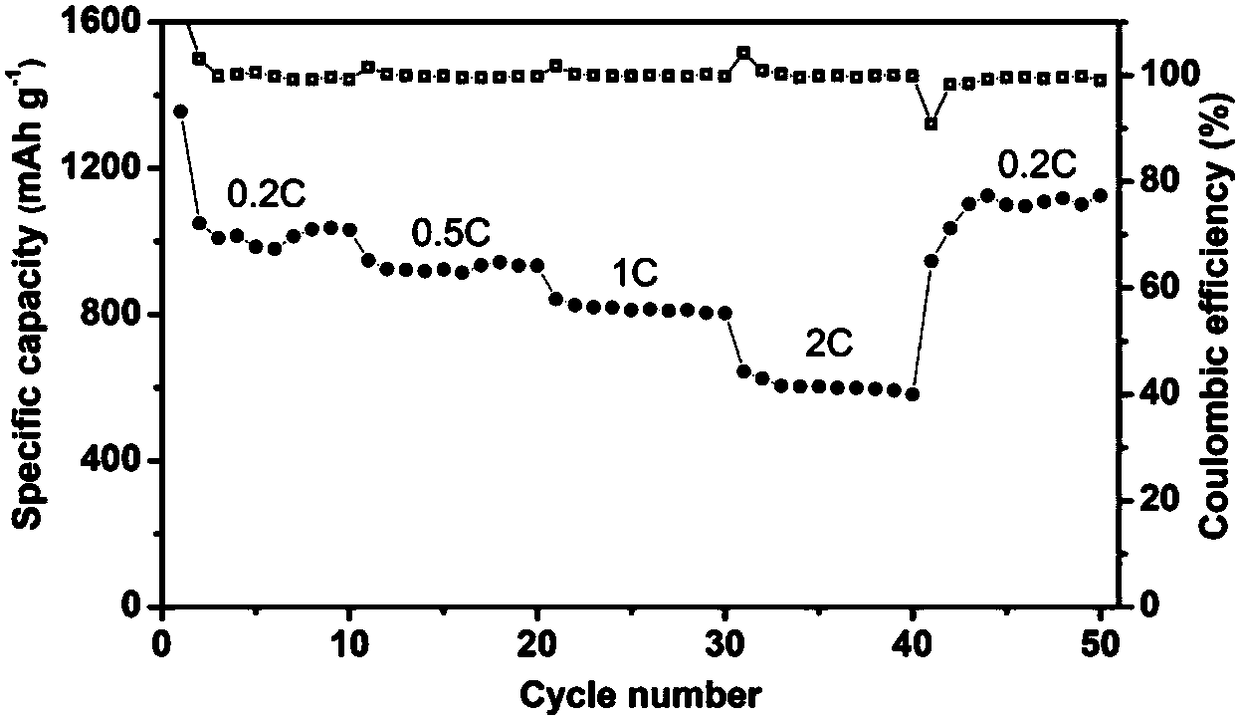
[0028] figure 1 The electrochemical rate performance diagram of the lithium-sulfur electrolyte prepared in Example 1 applied to the lithium-sulfur battery. It can be seen from the figure that the specific capacities of the battery for charge and discharge at 0.2, 0.5, 1 and 2C are 1049, 947, 842 and 644mAhg respectively -1 , when charging with a small rate of 0.2C, there is still 1100mAh g -1 , indicating that the battery has good rate performance and cycle stability.
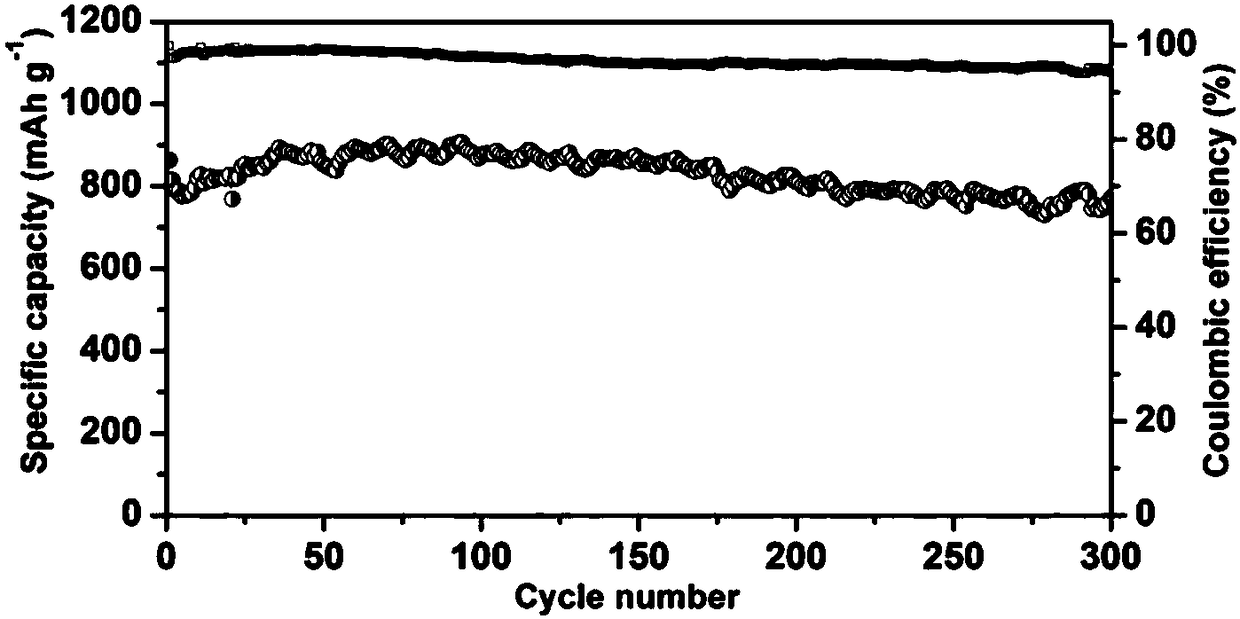
[0029] figure 2 The electrochemical cycle performance diagram of the lithium-sulfur electrolyte prepared for Example 1 applied to th...
Embodiment 2
[0031] This example is basically the same as Example 1, the only difference is that 0.1 g of carbon quantum dots is added to obtain a bright yellow electrolyte.
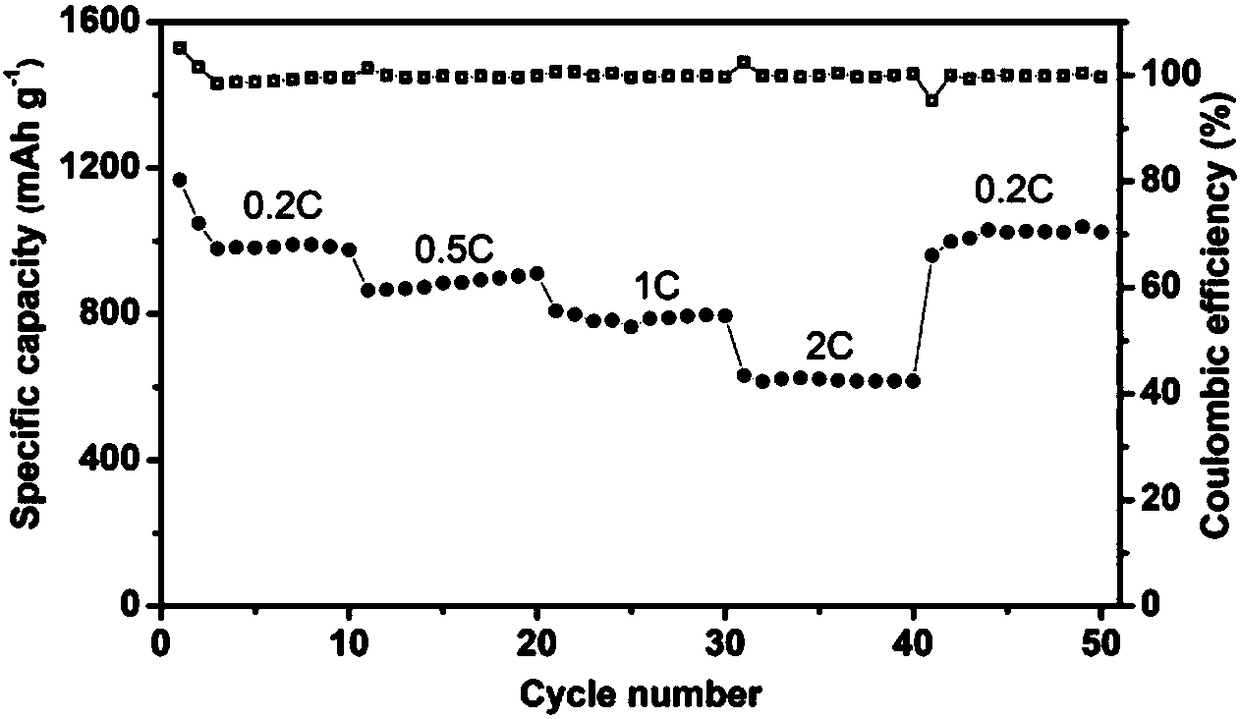
[0032] image 3 The electrochemical rate performance diagram of the lithium-sulfur electrolyte prepared for Example 2 applied to the lithium-sulfur battery. It can be seen from the figure that the specific capacities of the battery for charging and discharging at 0.2, 0.5, 1 and 2C are 1041, 853, 790 and 610mAhg respectively. -1 , when charging with a small rate of 0.2C, it is still stable at 1030mAh g -1 , indicating that the battery has good rate performance and cycle stability.
Embodiment 3
[0034] This embodiment is basically the same as Embodiment 1, the only difference is that 1g of carbon quantum dots is added to obtain a dark brown electrolyte.
[0035] Figure 4 The electrochemical rate performance diagram of the lithium-sulfur electrolyte prepared for Example 3 applied to the lithium-sulfur battery. It can be seen from the figure that the specific capacity of the battery for charging and discharging at 0.2, 0.5, 1 and 2C is 1000, 820, 760 and 650mAhg respectively -1 , when charging with a small rate of 0.2C, it is still stable at 910mAh g -1 , indicating that the battery has good rate performance and cycle stability.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com