Pharmaceutical composition for treating epileptiform convulsion, febrile convulsion and facial spasm and preparation method thereof
A technology for pediatric convulsion and hemifacial spasm, which is applied to a pharmaceutical composition for hemifacial spasm and its preparation. The drug is Xianyu Capsule, which can treat epilepsy convulsions and pediatric convulsions, and can solve frequent shutdowns for cleaning and capsule contents moisture absorption , accumulation and other problems, to achieve the effect of saving production costs and improving production efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Example 1 Preparation of medicinal material extract
[0029] The medicinal material extract of this embodiment is prepared by the following steps:
[0030] 1. Take Astragalus 1400g (here, 1 part by weight = 10g, the same below), Codonopsis 1400g, Salvia 1400g, Bupleurum 700g, Jujube seed 1050g, Polygala 700g, Gastrodia 1050g, Uncaria 1050g, Shichangpu 700g, Dannanxing 700g , Angelica 1400g, Stiff silkworm 1050g, Liushenqu 700g, Turmeric 700g, Licorice 700g, White Aconite 350g.
[0031] 2. Crush the dead silkworm and Liushenqu into fine powder, pass through a 80-100 mesh sieve, and set aside;
[0032] 3. Take the remaining fourteen medicinal materials such as Astragalus and decoct with water for three times, add 10 times the amount of water for the first time and cook for 2 hours, add 8 times the amount of water for the second time and cook for 1.5 hours, and add 6 times the amount of water for the third time 1 After hours, combine the decoction, filter, and concentrate the fil...
Embodiment 2
[0033] The screening test of the types of excipients in the pharmaceutical composition described in Example 2
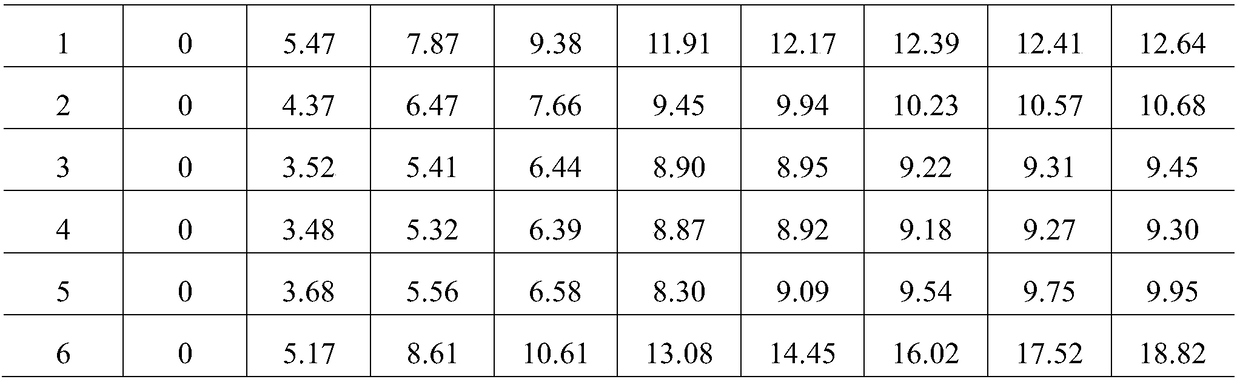
[0034] Take 50g of the drug extract prepared in Example 1 and divide it into 6 parts equally, and add the same weight (5g) of soluble starch, microcrystalline cellulose, calcium carbonate, dicalcium phosphate, lactose, and technical powder (stiff silkworm and Liushenqu Mix according to the ratio of 10.5:7, pulverize, sieving), and write them as 1# (soluble starch), 2# (microcrystalline cellulose), 3# (calcium carbonate), 4# (calcium hydrogen phosphate), 5# (Lactose), 6# (original powder), mix evenly, respectively weigh 2g accurately and spread them in a flat weighing bottle dried to constant weight, and then place it in a glass desiccator with a sodium chloride supersaturated solution at the bottom , Weigh accurately at 0, 4, 8, 12, 24, 36, 48, 72, 96h; make 3 copies of each auxiliary material in parallel, calculate the moisture absorption percentage% according to formu...
Embodiment 3
[0040] Example 3 Screening test of the proportion of auxiliary materials
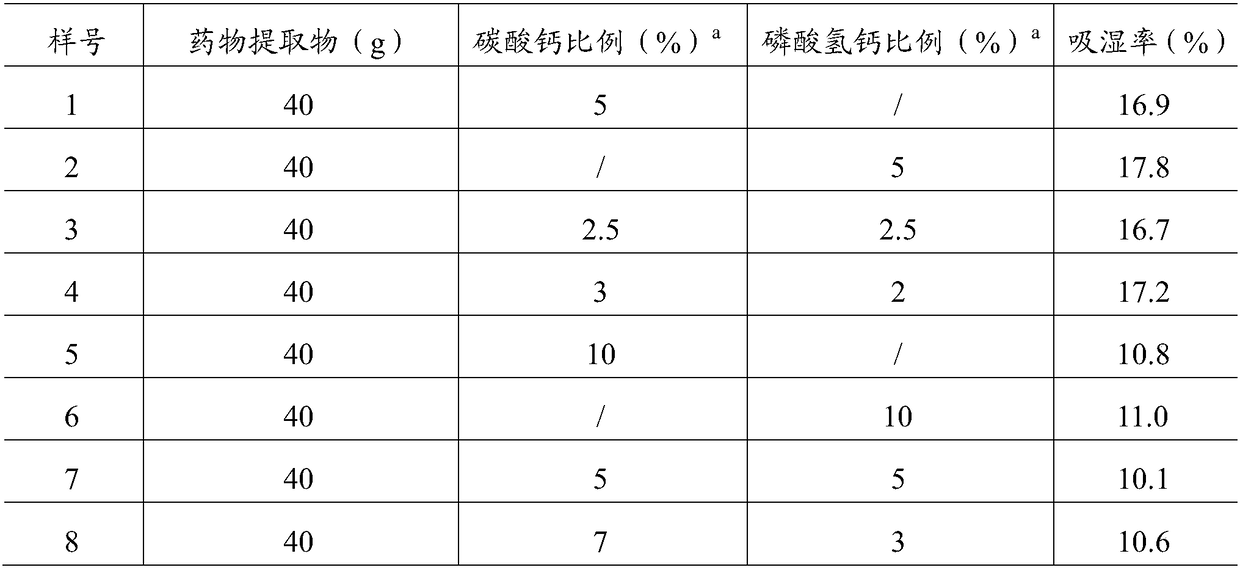
[0041] Take 8 parts of the drug extract prepared in Example 1 (40g each), add calcium carbonate and / or dibasic calcium phosphate in different weight ratios as shown in Table 2, and mix them evenly. Weigh 2g each and spread it evenly until it is dried. In a heavy flat weighing bottle, prevent it from being placed in a glass desiccator with a sodium chloride supersaturated solution at the bottom for 24h, take it out, and weigh again accurately; make 3 parts of each proportion of auxiliary materials in parallel, and calculate the moisture absorption percentage% according to formula A , Take the mean. The results are shown in Table 2.
[0042] Table 2 Screening test results of auxiliary material ratio / amount
[0043]
[0044] a : The ratio refers to the weight percentage of the drug extract.
[0045] The results in Table 2 show:
[0046] 1) The moisture absorption rate of the composition is obviously related to th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com