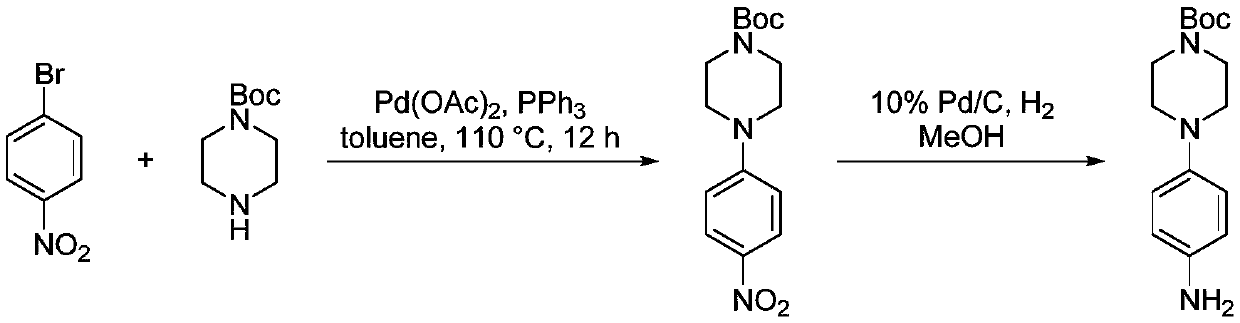
The preparation method of 4-(1-tert-butoxycarbonylpiperazin-4-yl)aniline
A technology of tert-butoxycarbonylpiperazine and aniline, which is applied in the field of preparation of 4-aniline, can solve the problems of low yield of target products, environmental and health hazards, and long synthesis routes, and avoid the use of heavy metals and hydrogen environments, Reduced production of by-products and low cost effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0010] The invention provides a preparation method of 4-(1-tert-butoxycarbonylpiperazin-4-yl)aniline. The preparation method of described 4-(1-tert-butoxycarbonylpiperazin-4-yl) aniline comprises the steps:
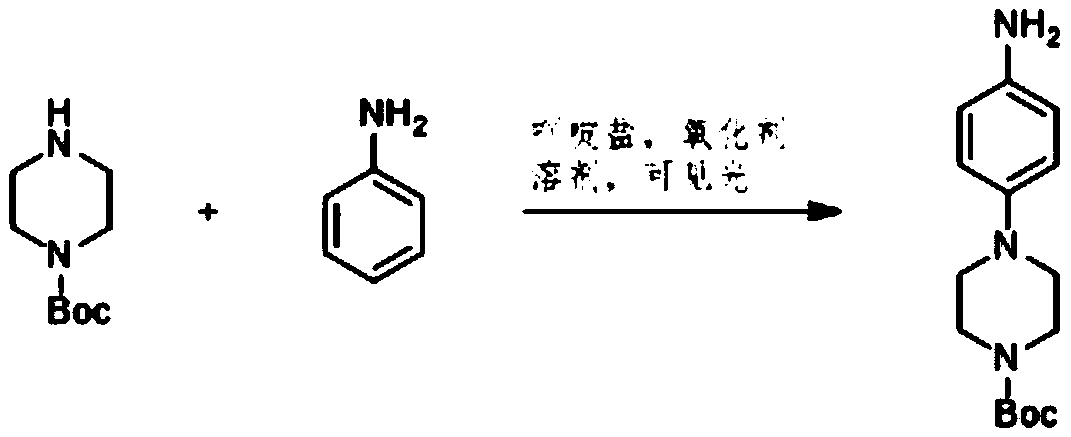
[0011] Add the aniline, piperazine-1-carboxylic acid tert-butyl ester, and acridinium salt photocatalyst into the solvent, and carry out light reaction under the condition of the presence of an oxidizing agent to generate 4-(1-tert-butoxycarbonylpiperazine-4 -yl) aniline.
[0012] Specifically, in the light reaction, the aniline and piperazine-1-carboxylic acid tert-butyl ester generate the product compound 4-( 1-tert-butoxycarbonylpiperazin-4-yl)aniline. The chemical reaction formula of the light reaction is as follows:
[0013]
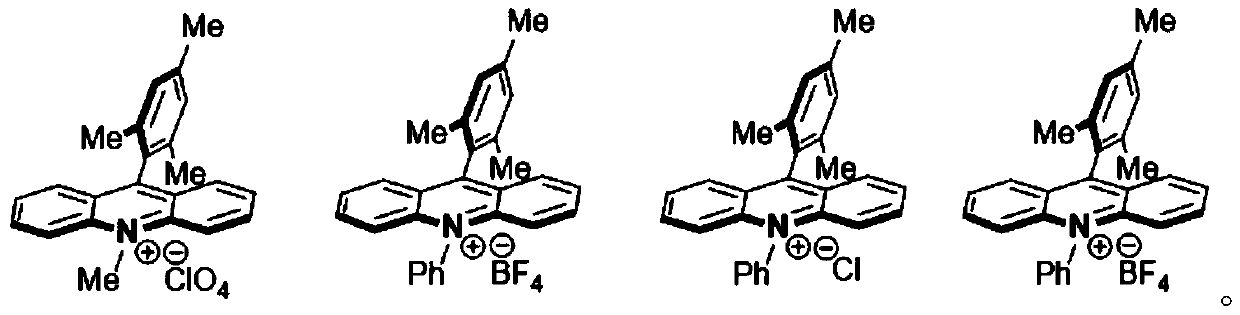
[0014] Wherein, the acridinium salt promotes the condensation reaction of the aniline and tert-butyl piperazine-1-carboxylate under light conditions. In one embodiment, the acridinium salt photocatalyst includes at least one of the follo...
Embodiment 1
[0025] This example provides a preparation method of 4-(1-tert-butoxycarbonylpiperazin-4-yl)aniline. The synthetic method of described 4-(1-tert-butoxycarbonylpiperazin-4-yl)aniline:
[0026] Add aniline, piperazine-1-carboxylic acid tert-butyl ester, acridinium salt visible light catalyst, 2,2,6,6-tetramethylpiperidine-nitrogen-oxide into anhydrous dichloroethane, and then use oxygen The reaction environment was replaced three times, irradiated with a blue LED, and the reaction time was 10 h. After the reaction was completed, the filtrate was spin-dried and separated by column chromatography to obtain the target product as a colorless white solid with a yield of 95%.
[0027] Among them, aniline, piperazine-1-carboxylic acid tert-butyl ester, acridinium salt, 2,2,6,6-tetramethylpiperidine-nitrogen-oxide and anhydrous dichloroethane are added according to the following ratio: For every 2mL of anhydrous dichloroethane, add 0.2mmol, 1.0eq of aniline, 0.2mmol, 1.0eq of tert-but...
Embodiment 2
[0032] This example provides a preparation method of 4-(1-tert-butoxycarbonylpiperazin-4-yl)aniline. The synthetic method of described 4-(1-tert-butoxycarbonylpiperazin-4-yl)aniline:
[0033] Add aniline, piperazine-1-carboxylic acid tert-butyl ester, acridinium salt visible light catalyst, 2,2,4,6,6-pentamethylpiperidine-nitrogen-oxide into anhydrous dichloroethane, and then The reaction environment was replaced by oxygen three times, irradiated with a blue LED, and the reaction time was 10 h. After the reaction was completed, the filtrate was spin-dried and separated by column chromatography to obtain the target product as a colorless white solid with a yield of 93%.
[0034]Among them, aniline, piperazine-1-carboxylic acid tert-butyl ester, acridinium salt, 2,2,4,6,6-pentamethylpiperidine-nitrogen-oxide and anhydrous dichloroethane follow the following ratio Addition: Add 0.2mmol, 1.0eq of aniline, 0.2mmol, 1.0eq of tert-butyl piperazine-1-carboxylate, 0.01mmol, 0.1eq of ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com