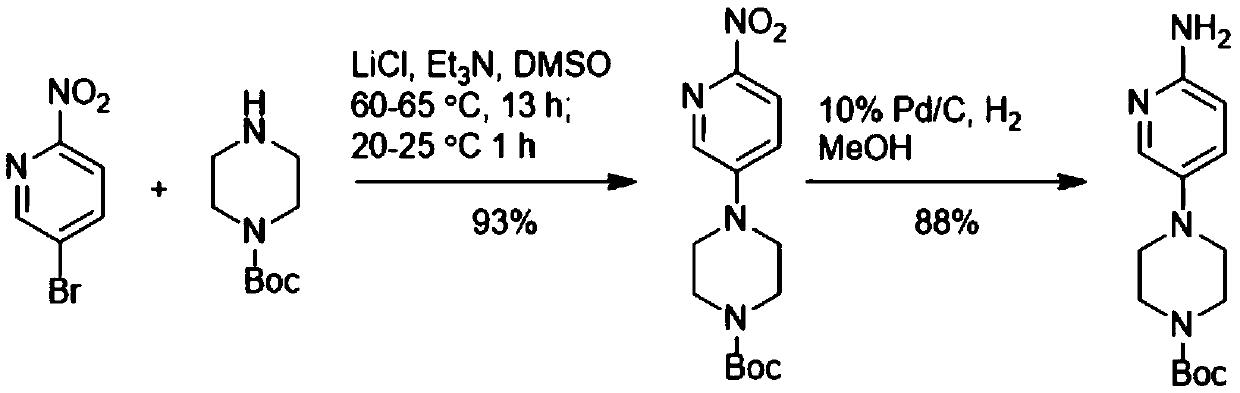
The preparation method of 4-(6-aminopyridin-3-yl)piperazine-1-carboxylic acid tert-butyl ester
A technology of tert-butyl carboxylate and aminopyridine is applied in the field of preparation of tert-butyl 4-piperazine-1-carboxylate, and can solve the problems of low yield of target product, many by-products, less than comprehensive yield and the like , to avoid the use of heavy metals and hydrogen environment, reduce the generation of by-products, and shorten the synthesis path.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0011] The invention provides a preparation method of tert-butyl 4-(6-aminopyridin-3-yl)piperazine-1-carboxylate. The preparation method of described 4-(6-aminopyridin-3-yl) piperazine-1-carboxylic acid tert-butyl ester comprises the steps:
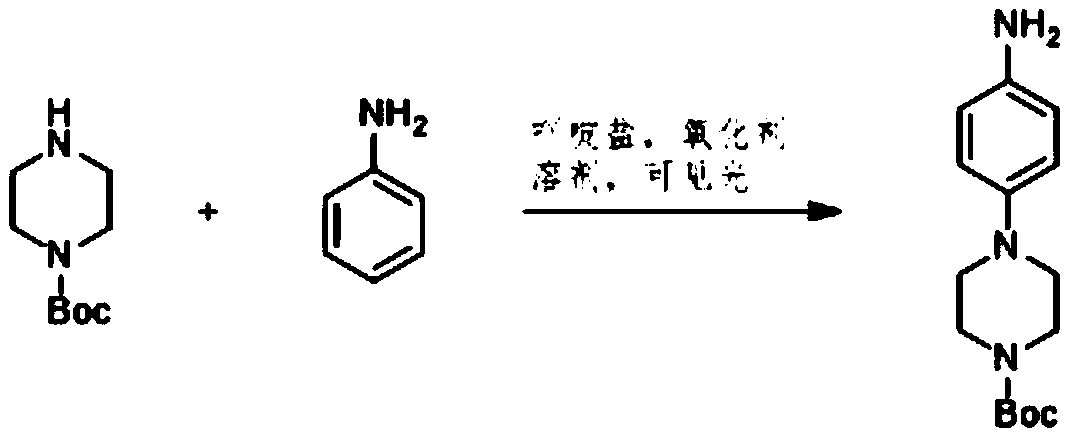
[0012] Add 2-aminopyridine, piperazine-1-carboxylic acid tert-butyl ester, and acridinium salt photocatalyst into the solvent, and carry out light reaction under the condition of oxidizing agent to generate 4-(6-aminopyridin-3-yl) tert-Butyl piperazine-1-carboxylate.
[0013] Specifically, in the light reaction, the 2-aminopyridine and piperazine-1-carboxylic acid tert-butyl ester generate the product compound c. The chemical reaction formula of the light reaction is as follows:
[0014]
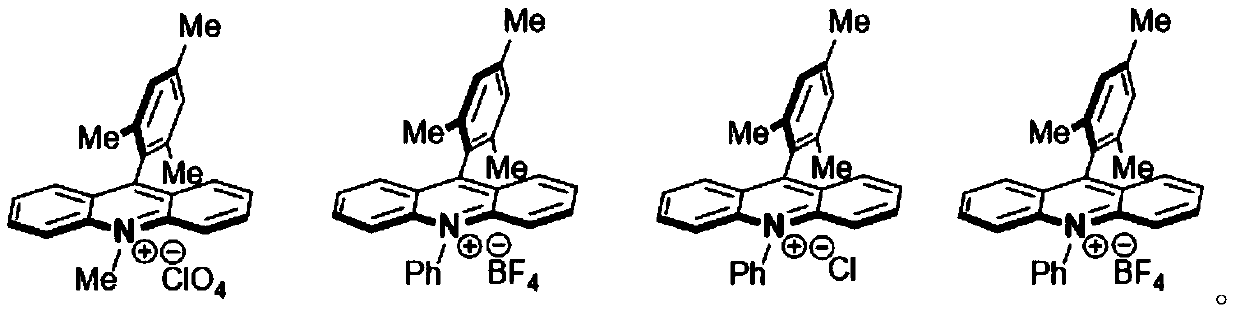
[0015] Wherein, the acridinium salt promotes the condensation reaction of the 2-aminopyridine and piperazine-1-carboxylic acid tert-butyl ester under light conditions. In one embodiment, the acridinium salt photocatalyst includes at least one of ...
Embodiment 1
[0026] This example provides a preparation method of tert-butyl 4-(6-aminopyridin-3-yl)piperazine-1-carboxylate. The synthetic method of described 4-(6-aminopyridin-3-yl) piperazine-1-carboxylic acid tert-butyl ester:
[0027] Add 2-aminopyridine, piperazine-1-carboxylic acid tert-butyl ester, acridinium salt visible light catalyst, 2,2,6,6-tetramethylpiperidine-nitrogen-oxide into anhydrous dichloroethane, Then, the reaction environment was replaced with oxygen three times, irradiated with a blue LED, and the reaction time was 10 h. After the reaction was completed, the filtrate was spin-dried and separated by column chromatography to obtain the target product as a colorless white solid with a yield of 95%.
[0028] Wherein, the 2-aminopyridine, piperazine-1-carboxylic acid tert-butyl ester, acridinium salt, 2,2,6,6-tetramethylpiperidine-nitrogen-oxide and anhydrous dichloroethane according to Add in the following ratio: for every 2mL of anhydrous dichloroethane, add 0.2mmo...
Embodiment 2
[0033] This example provides a preparation method of tert-butyl 4-(6-aminopyridin-3-yl)piperazine-1-carboxylate. The preparation method of described m-aminophenylacetylene is as follows:
[0034] Add 2-aminopyridine, piperazine-1-carboxylate tert-butyl ester, acridinium salt visible light catalyst, 2,2,4,6,6-pentamethylpiperidine-nitrogen-oxide into anhydrous dichloroethane , and then replace the reaction environment with oxygen three times, irradiate with blue LED, and the reaction time is 10h. After the reaction was completed, the filtrate was spin-dried and separated by column chromatography to obtain the target product as a colorless white solid with a yield of 92%.
[0035] Among them, the 2-aminopyridine, piperazine-1-carboxylic acid tert-butyl ester, acridinium salt, 2,2,4,6,6-pentamethylpiperidine-nitrogen-oxide and anhydrous dichloroethyl Alkanes were added according to the following ratio: for every 2mL of anhydrous dichloroethane, 0.2mmol, 1.0eq of 2-aminopyridine...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com