Preparation method of deuterium-labeled betamethasone
A technology of betamethasone and deuterium labeling, which is applied in the fields of organic chemistry methods, chemical instruments and methods, steroid isotope introduction, etc., can solve the problems such as no synthesis report of betamethasone deuterium-labeled compounds, and achieve easy labeling and good stability , the effect of high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
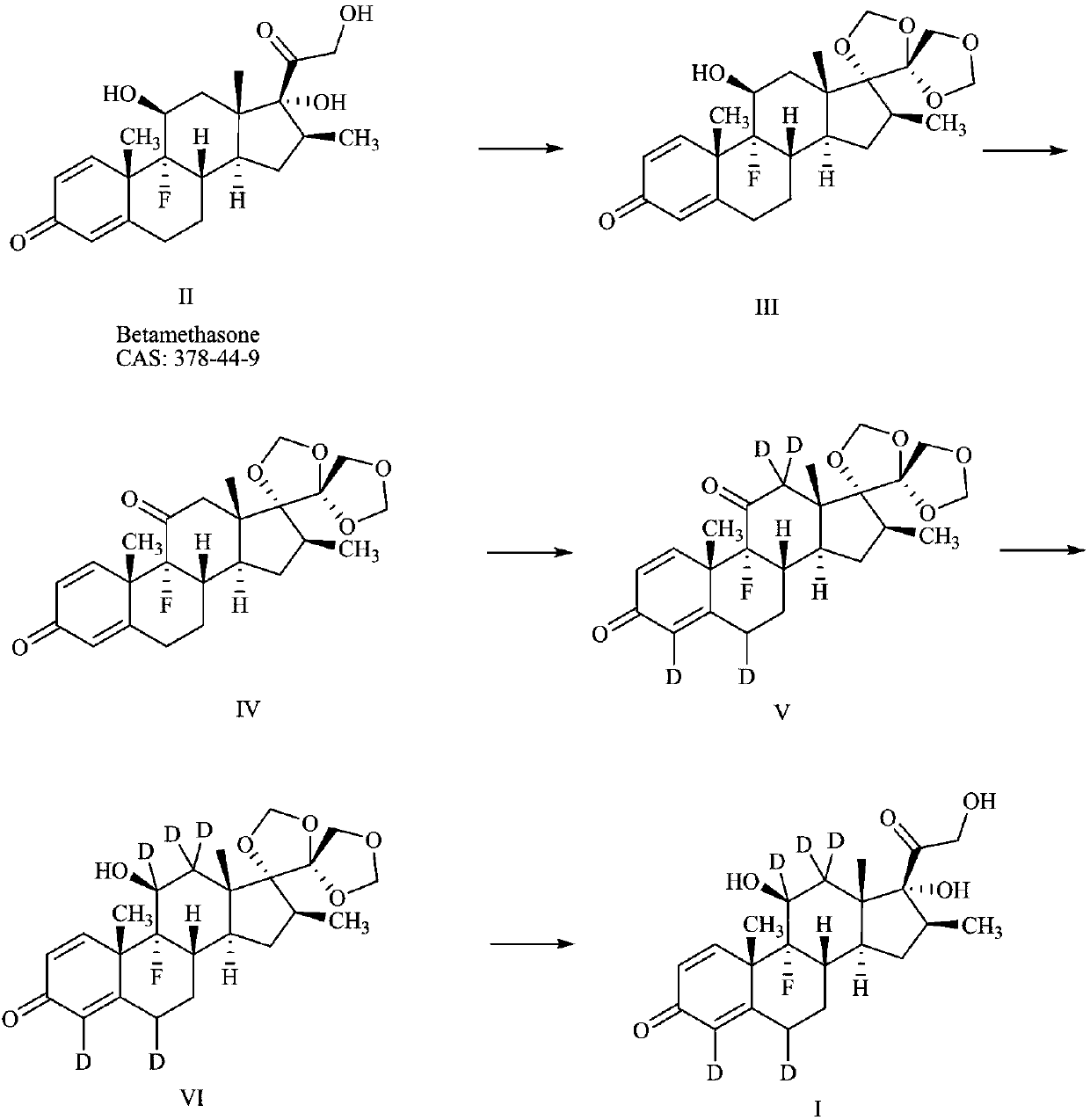
[0035] A preparation method of deuterium-labeled betamethasone, such as figure 1 shown, including the following steps:
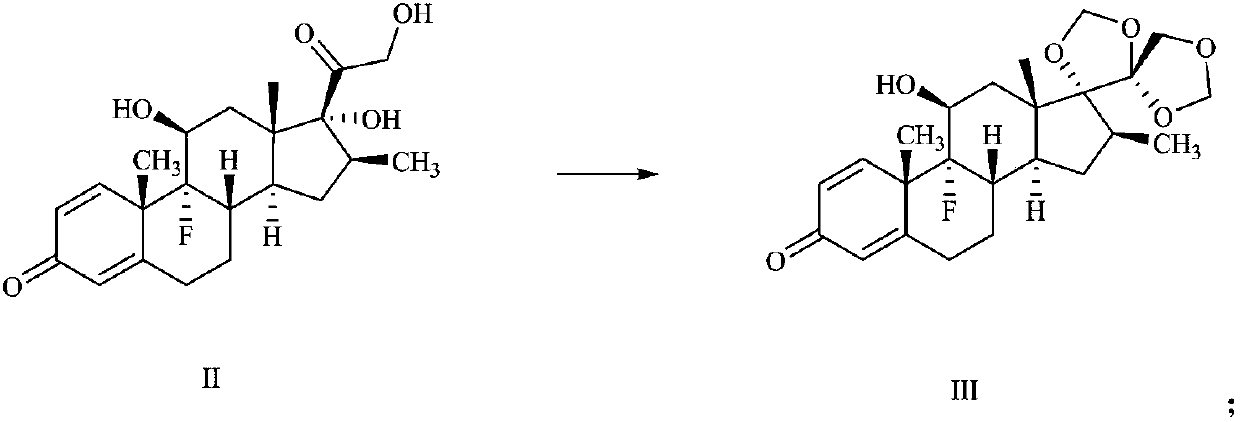
[0036] (1) Preparation of compound III:
[0037]
[0038] 30 g of betamethasone (compound II) was suspended in 400 ml of chloroform and 600 ml of 7 mol / L hydrochloric acid mixed solvent, and 64.21 g of paraformaldehyde was added in an ice bath. The reaction mixture was stirred at 30°C for 3 hours, the reaction solution was extracted three times with 300 ml of chloroform, and spin-dried to obtain 30.2 g of crude product, which was crystallized with methanol and dichloromethane to obtain 28 g of compound III, with a yield of 84.4%, MS: 435.5 [M+ H] + . 1 H NMR (400MHz, CDCl 3 ):δ0.88(s,3H),1.1(m,4H),1.52(s,3H),1.60~2.7(9H),3.35(m,1H),3.99~4.18(dd,2H),4.35( s,1H),4.85(s,1H),5.07(d,2H),5.13(s,1H),6.17(s,1H),6.27(d,1H),7.45(d,1H).
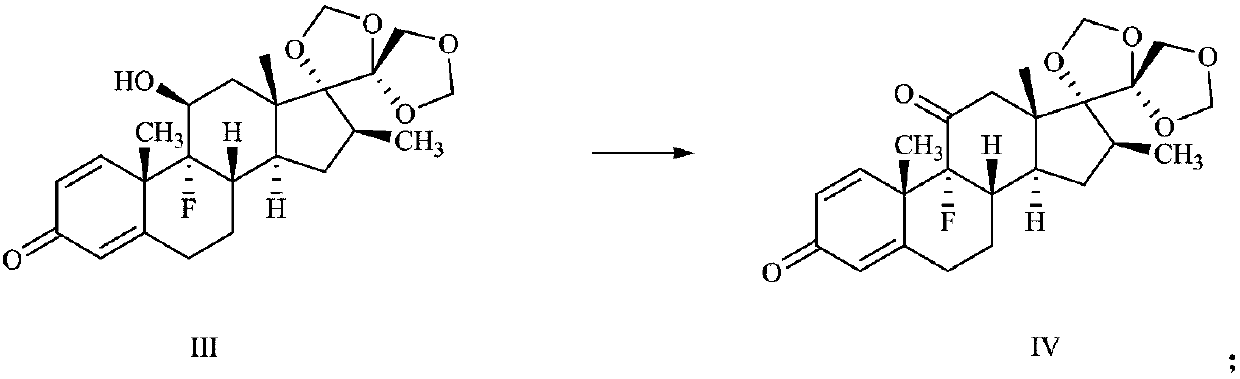
[0039] (2) Preparation of Compound IV:
[0040]
[0041] Suspend 10 g of compound III in 400 ml of dichloromethane, add...
Embodiment 3
[0074] Anti-inflammatory and anti-allergic experiments of embodiment 3 deuterium-labeled betamethasone
[0075] 1. Xylene-induced mouse auricle swelling experiment (mouse ear swelling method)
[0076] Get 60 mice, be divided into 7 groups at random, namely blank control (normal saline) group, model group, aspirin group and deuterium-labeled betamethasone (compound I) (0.05g / ml) intragastric administration, continuous 3d, The volume of administration is 0.1ml / 10g. One hour after the last administration, smear 0.05ml of xylene on both sides of the right ear of the mouse evenly, and the left ear is not applied as a control. Ear pieces were punched at the same position in both ears, and the weight of the ear pieces was weighed on an analytical balance. The difference in weight was used as the degree of swelling, and the swelling inhibition rate was calculated.
[0077] Ear swelling (%) = (right ear weight - left ear weight) / left ear weight × 100%
[0078] Inhibition rate of ea...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com