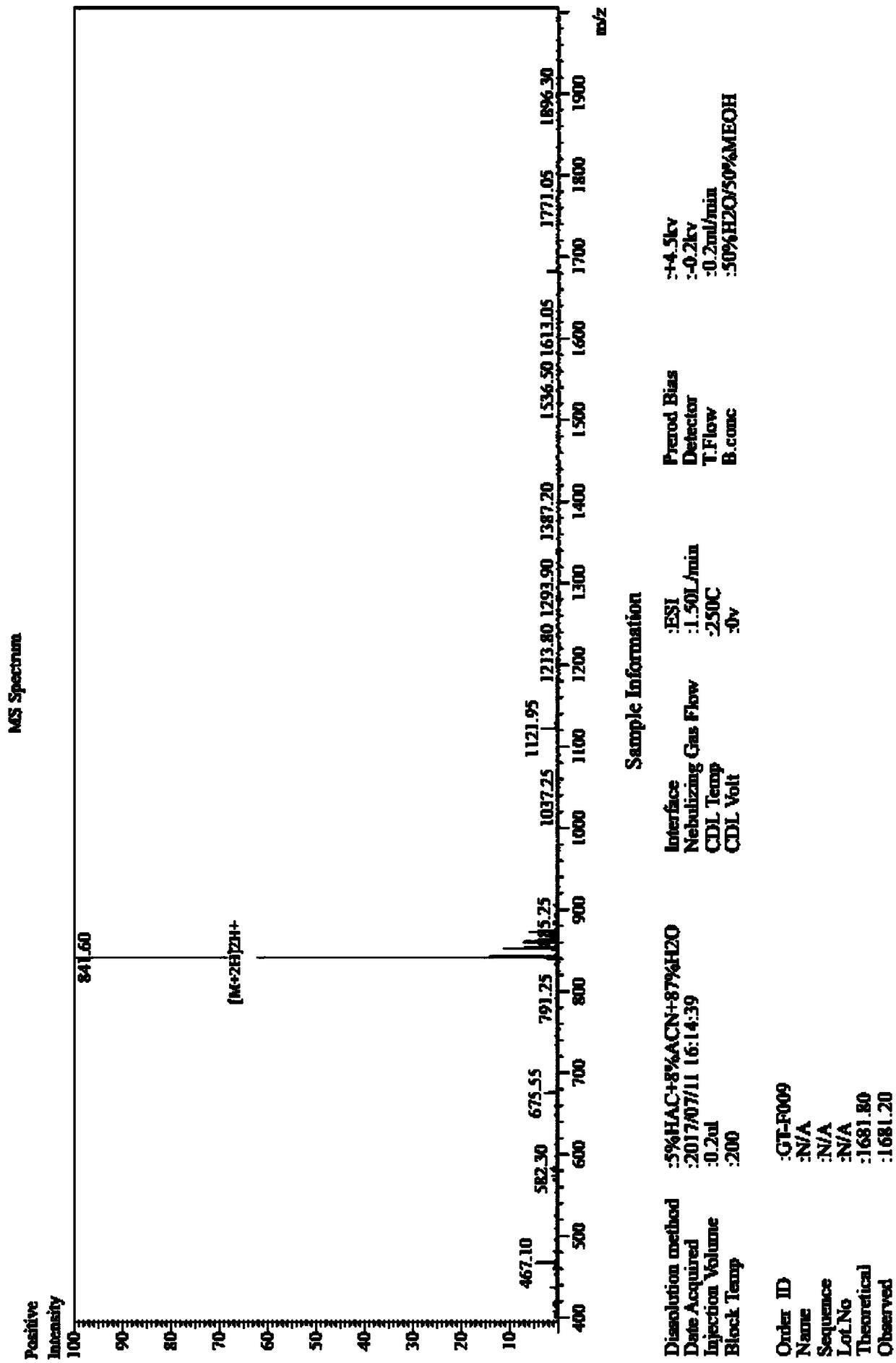
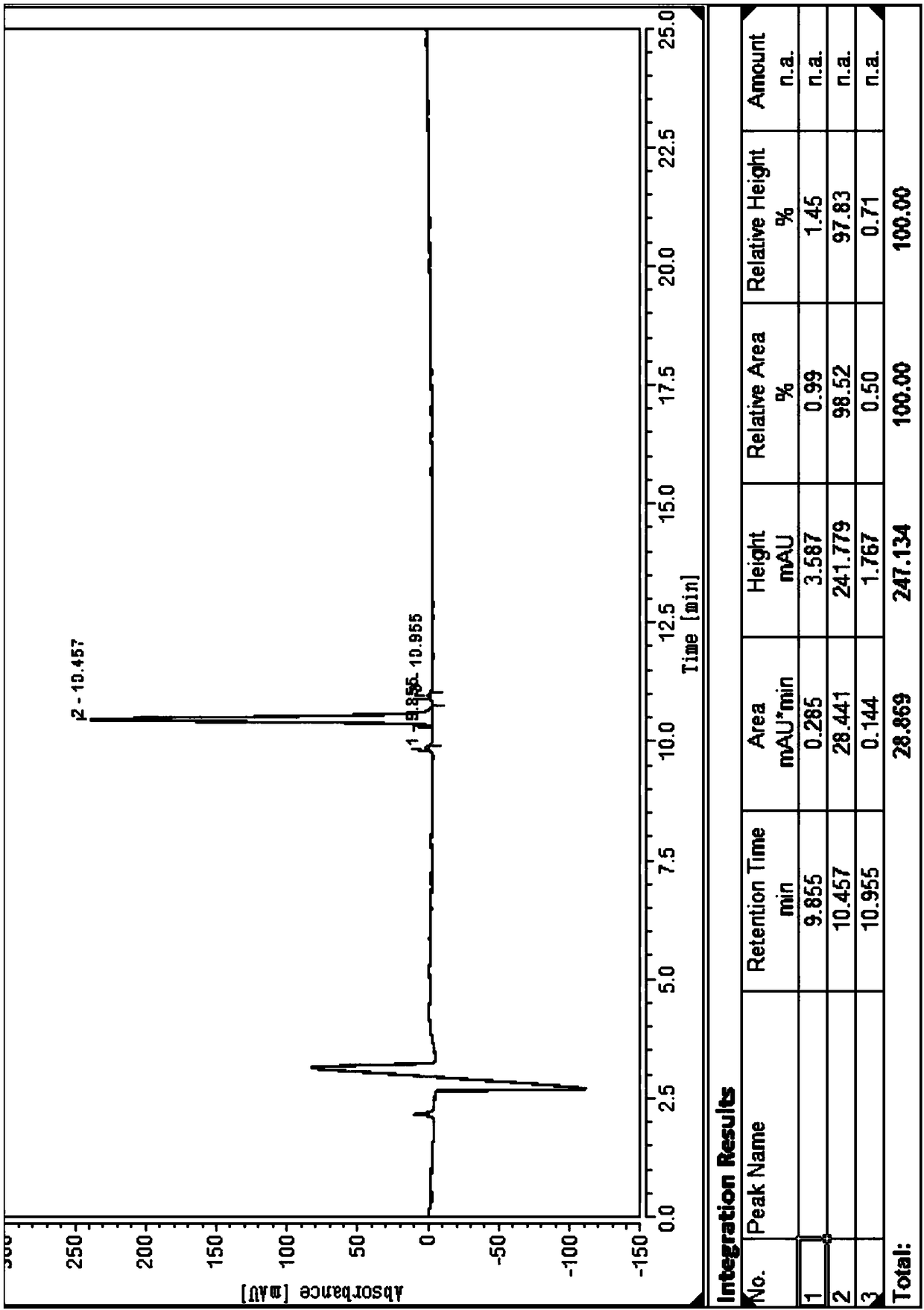
Solid phase synthetic method of plecanatide
A plecanatide and solid-phase synthesis technology, which is applied in the field of solid-phase synthesis of plecanatide, can solve problems such as inability to accurately position, achieve the effects of guaranteed yield, real-time controllable cyclization process, and improved efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Embodiment 1: a kind of solid phase synthesis method of plecanatide, comprises the following steps:
[0025] (1) Use catalytic esterification conditions to serially load Fmoc-Leu-OH onto WANG resin;
[0026] (2) According to the plecanatide sequence, use a condensation reagent to sequentially couple and protect amino acids from the C-terminal to the N-terminal, wherein the plecanatide sequence sequence is: Asn(trt)16-Asp(tbu)15-Glu(otbu) 14-Cys(trt)13-Glu(otbu)12-Leu11-Cys(A cm)10-Val9-Asn(trt)8-VaL7-Ala6-Cys(trt)5-Thr(tbu)4-Gly3-Cys (Acm)2-Le u1-wang resin;
[0027] (3) Cleavage the polypeptide from the resin with a TFA cleavage reagent to obtain free polypeptides with Acm protection at the 2 and 10 positions, in which the sulfhydryl groups of the other pair (5, 13 positions) Cys have been exposed; under alkaline conditions, Use 30% hydrogen peroxide / hydrogen peroxide with a reaction solution volume of 0.5ml / g-1ml / g to oxidize the pair of sulfhydryl groups to obtain ...
Embodiment 2
[0038] Embodiment 2: Synthesis method of plecanatide linear chain crude peptide
[0039] 1. Preparation of resin: Fmoc-Leu-Wang Resin 6mmol with a substitution degree (loading) of 0.35
[0040] Accurately weigh 26.5g of Fmoc-Leu-OH, 10.1g of Hobt, and 0.92g of Dmap into the Erlenmeyer flask, and dissolve with a small amount of DMF; then weigh 85.7g of Wang resin with an original Loading of 0.96 into the Erlenmeyer flask, and adjust to an appropriate reaction with DMF Concentration, add 11.6ML of DIC, temperature control 28-30 ℃, shaker reaction 3h. Remove the solution with a sand core reaction column, wash with DMF, DCM, and DMF twice respectively, add 155.6ml of acetic anhydride and 132.4ml of pyridine, use DMF as the reaction solvent, and seal at a temperature of 28-30°C for 5 hours. After the sealing is completed, the solution is removed, washed three times with DMF, DCM, and methanol in turn, and dried for later use.
[0041] The resin substitution degree measured by ult...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Degree of substitution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com