Drug composition for removing salmonella in breeding birds
A composition and technology for Salmonella, which can be used in drug combinations, antibacterial drugs, pharmaceutical formulations, etc., and can solve problems such as limited protective efficacy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
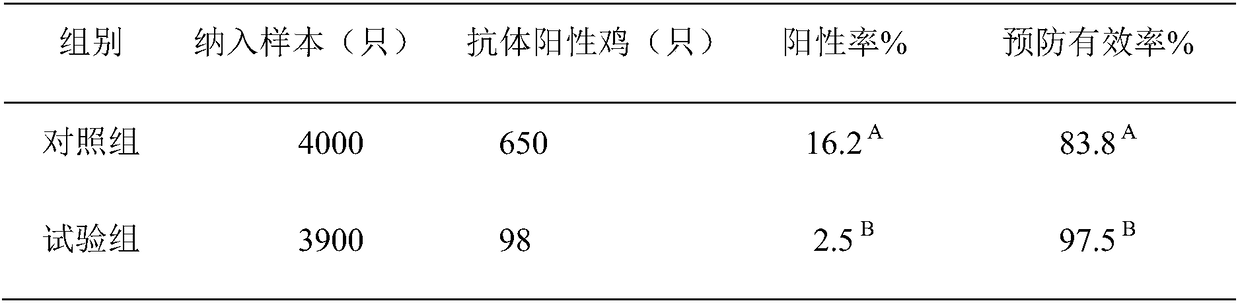
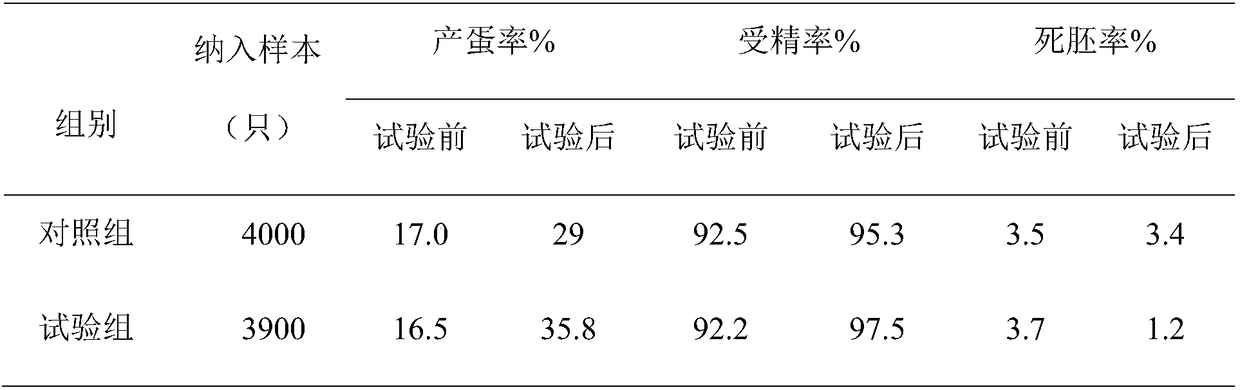
Examples
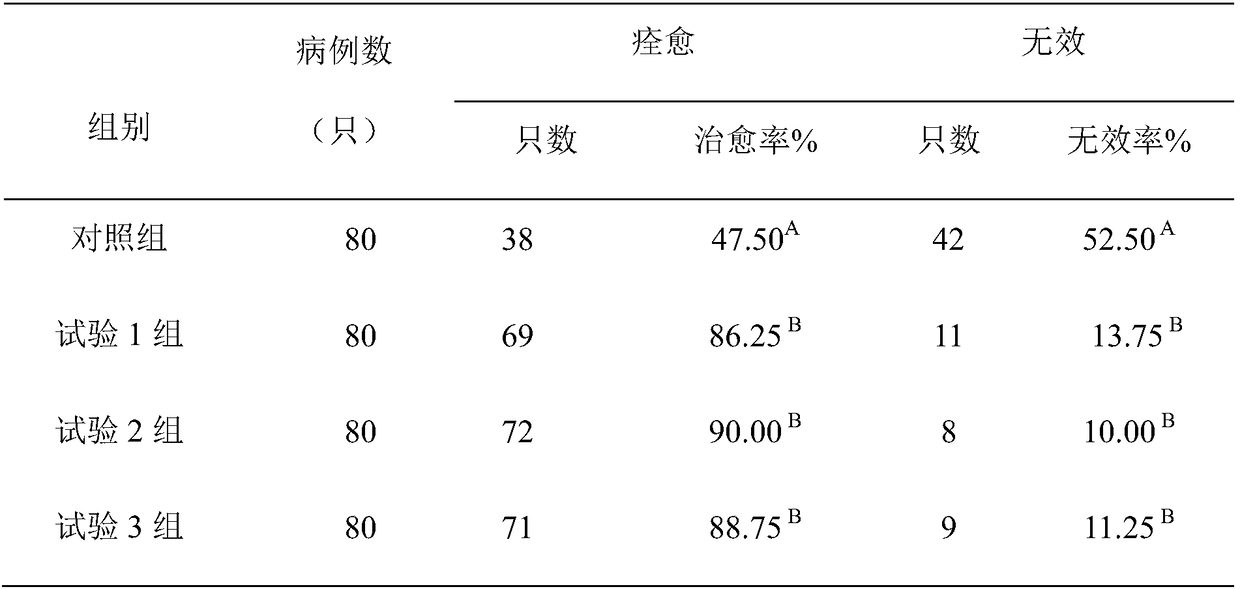
Embodiment 1
[0029] The formula of the pharmaceutical composition of purifying breeder Salmonella of the present invention is:
[0030] 30 parts by weight of baicalin flavone, 20 parts by weight of L-lysine, and 30 parts by weight of L-arginine.
[0031] Wherein, the preparation method of Scutellaria baicalensis is:
[0032] (1) Extraction of Scutellaria baicalensis flavonoids: crush the decoction pieces of Scutellaria baicalensis, pass through an 80-mesh sieve, add 5 times the weight of medicinal materials and 50% dimethyl sulfoxide solution, extract twice at 40°C for 60 minutes each time, filter, and combine the filtrates, spare.
[0033] (2) Membrane separation: the filtrate obtained in step (1) is subjected to membrane separation, and the membrane separation conditions are: select 10nm inorganic ceramic membrane, flow rate is 1.5L / min, pressure 0.12MPa and feed liquid temperature 30°C, to obtain Scutellaria baicalensis Concentrate.
[0034] (3) Spray drying: the baicalin flavone con...
Embodiment 2
[0038] The formula of the pharmaceutical composition of purifying breeder Salmonella of the present invention is:
[0039] 35 parts by weight of Scutellaria baicalensis, 30 parts by weight of L-lysine, and 35 parts by weight of L-arginine.
[0040] The preparation method of baicalin flavonoids is the same as in Example 1.
[0041] According to the prescription proportion, 35 kg of baicalin flavonoids, 30 kg of L-lysine and 35 kg of L-arginine were weighed, mixed evenly and subpackaged to obtain a pharmaceutical composition for purifying Salmonella chicken breeder.
Embodiment 3
[0043] The formula of the pharmaceutical composition of purifying breeder Salmonella of the present invention is:
[0044] 50 parts by weight of baicalin flavone, 40 parts by weight of L-lysine, and 50 parts by weight of L-arginine.
[0045] The preparation method of baicalin flavonoids is the same as in Example 1.
[0046] According to the prescription ratio, 50 kg of baicalin flavones, 40 kg of L-lysine, and 50 kg of L-arginine are weighed, mixed evenly, and packaged separately to obtain a pharmaceutical composition for purifying Salmonella chicken breeder.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com