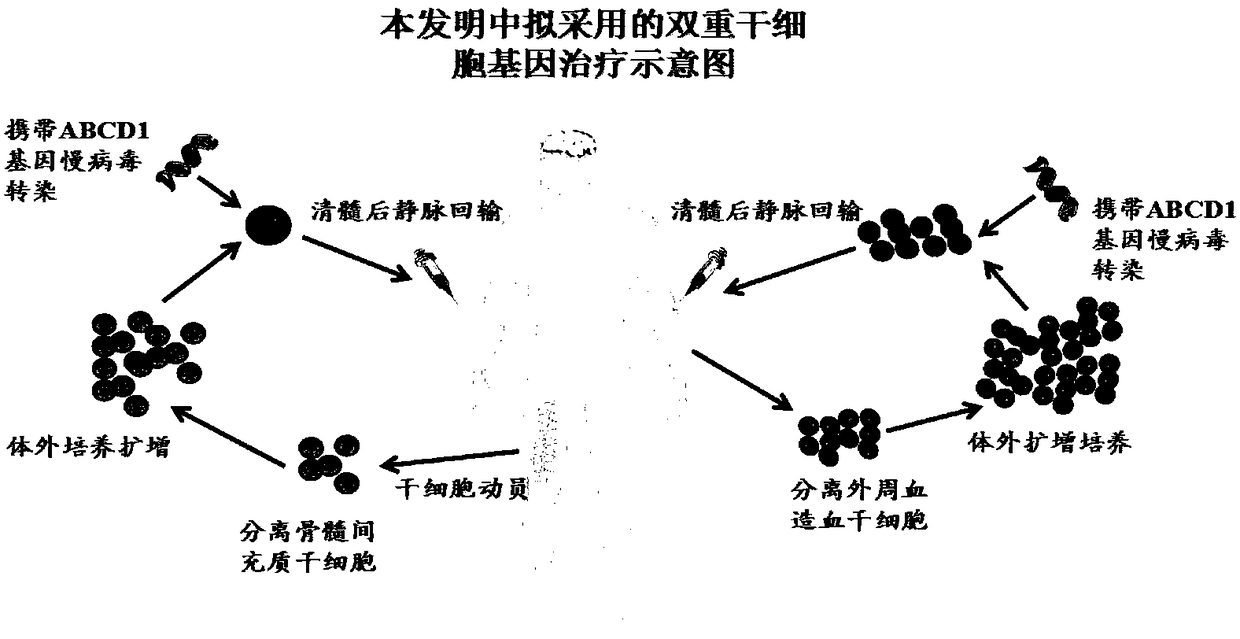
Method for treating adrenoleukodystrophy (ALD) through optimized expression of ABCD1 gene with lentiviral vector EF1alpha promoter
A technology of lentiviral vector and promoter sequence, which is applied in the field of genetic engineering, can solve the problems that the clinical effect of disease treatment cannot meet expectations, patients lose the ability to move and speak, and the difference in gene transfer efficiency, etc., to achieve comprehensive and durable gene therapy, The effect of ensuring safety and efficient gene delivery
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015] Example 1: Construction of a lentiviral vector carrying the normal ABCD1 gene
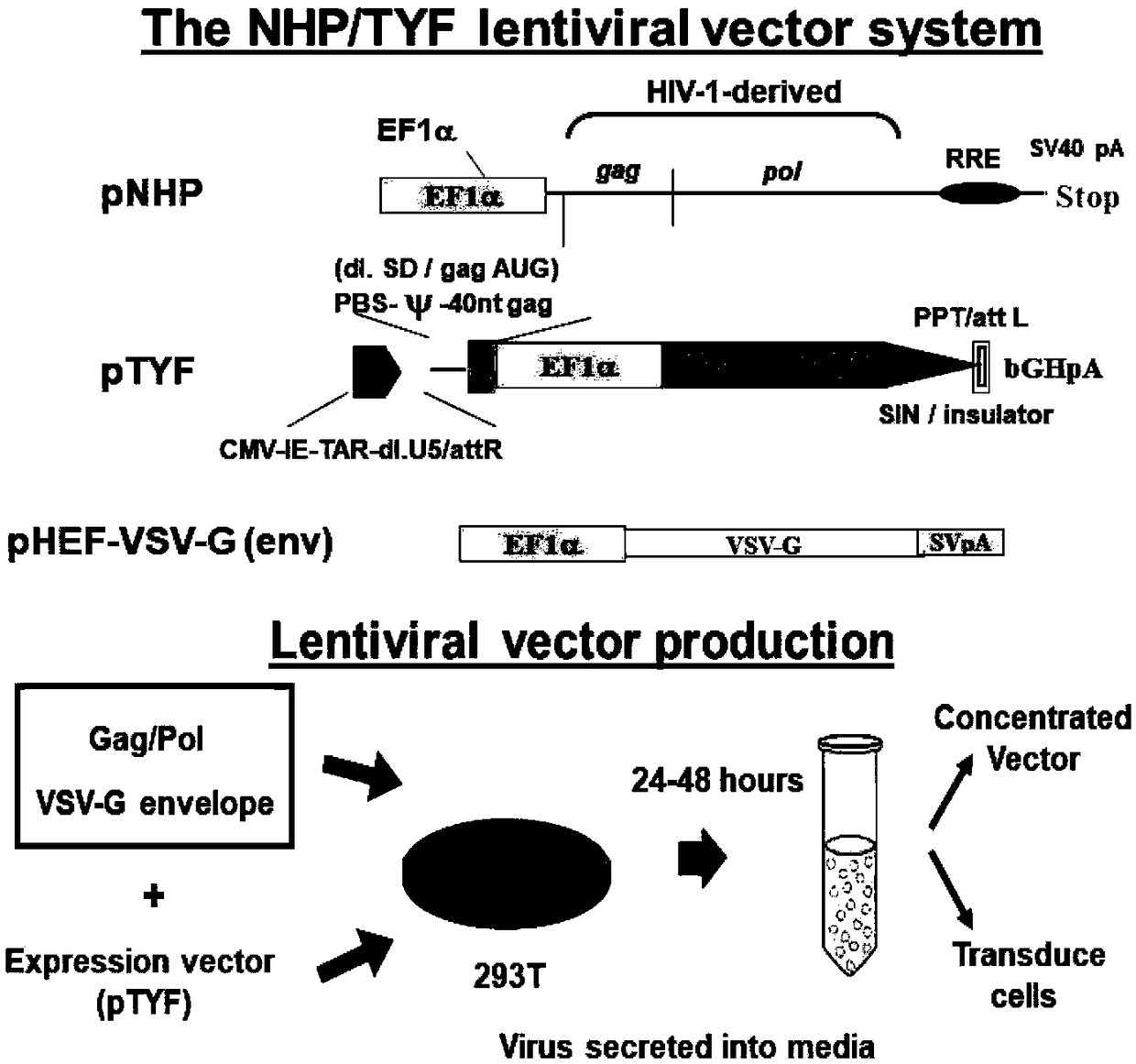
[0016] Synthesize the normal ABCD1 gene sequence (as shown in SEQ ID NO.1) and the human EF1α promoter sequence (as shown in SEQ ID NO.3) through the whole gene, and connect it into the lentiviral vector ( In NHP / TYFlentivirus vector system), the obtained product was identified by sequencing and double enzyme digestion (5' using BamHI cloning siteggatccacc-AUG; 3' using SpeI site for cloning, and the best reaction conditions refer to the original NEB factory recommendation) and other methods. A lentiviral expression vector (as shown in SEQ ID NO.3) carrying the normal ABCD1 gene under the promoter of correctly connected hEF1α was obtained. The specific connection position and lentiviral vector composition are as follows: figure 1 shown.
[0017] Packing, purification and concentration of lentivirus for protein identification after transfection of stem cells The protein expression of ABCD1 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com