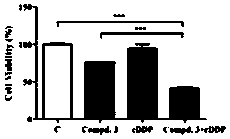
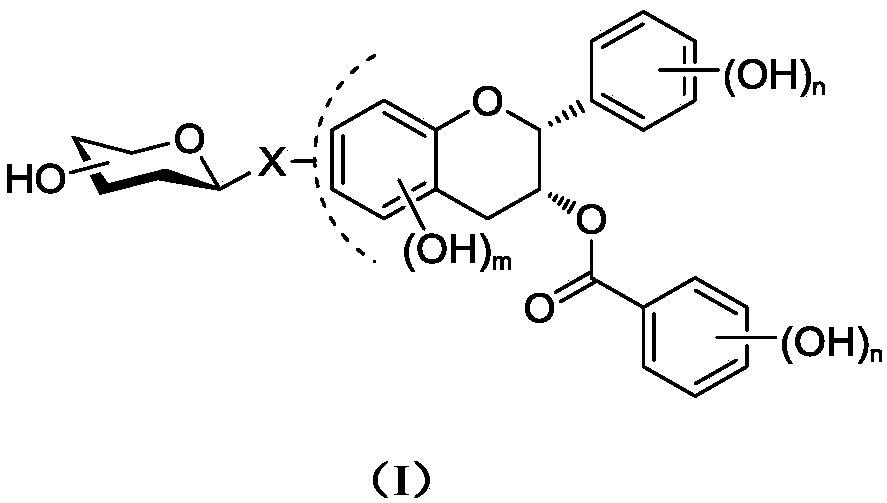
Epigallocatechin gallate glycoside derivative and application thereof
A technology of epigallocatechin and glycoside derivatives is applied in the application field of preparing anti-cancer preparations, and can solve the problems of low bioavailability, insufficient stability, affecting the efficacy of cancer treatment and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
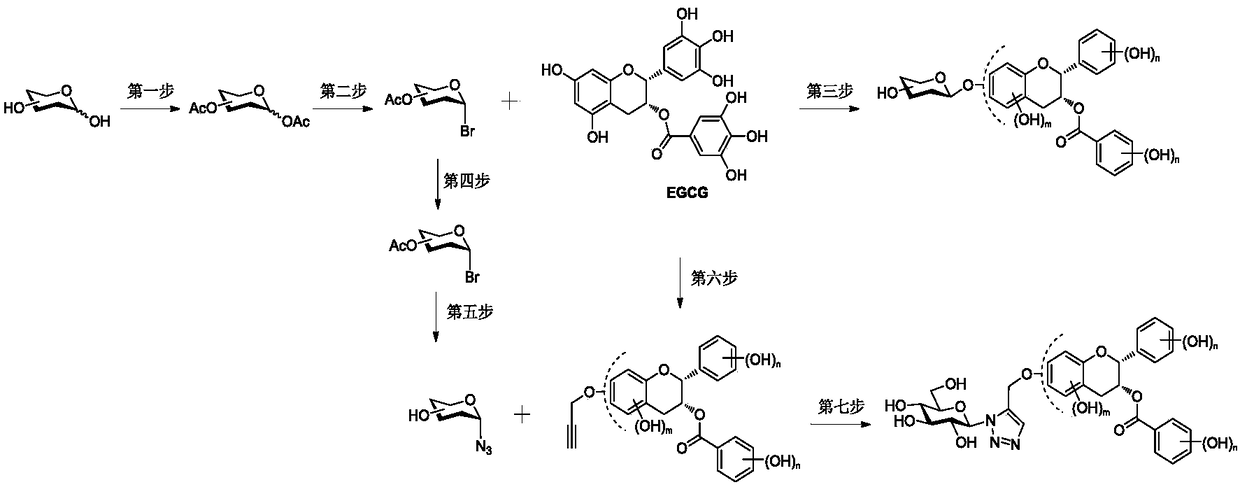
[0045] The synthesis process of the present invention is as figure 2 shown.
Embodiment 2
[0047] Preparation of compound 4"-β-glucoside epigallocatechin gallate (Compd.1) (steps 1, 2, and 3):
[0048] first step: Preparation of 1,2,3,4,6-peracetyl-β-glucose:
[0049] Dissolve D-glucose (3.6g, 20mmol) in acetic anhydride (8.3mL, 70mmol), heat and stir in an oil bath preheated to 100°C, then slowly add anhydrous sodium acetate (2.4g, 60mmol ), the reaction was terminated after heating to reflux at 100° C. for 2 h. After the reaction was completed, add water to dilute the reaction solution and extract three times with saturated sodium bicarbonate solution, then extract three times with water, extract the organic phase, dry over anhydrous sodium sulfate and concentrate under reduced pressure to obtain a crude product purified by silica gel chromatography (elution condition: petroleum ether / ethyl acetate=1:1). 2.62 g of compound 1,2,3,4,6-peracetyl-β-glucose was obtained with a yield of 72%. 1 H-NMR (CDCl 3 ,400MHz)δ5.70(d,1H,J=8.4Hz,C1-H),5.74(t,1H,J=9.2Hz,C 3 -...
Embodiment 3
[0056] Preparation of 5-(1,2,3-triazole-β-D-glucoside) epigallocatechin gallate (Compd.3) (steps 1, 2 and steps 4, 5, 6, and 7 ):
[0057] the fourth step: Preparation of azide-2,3,4,6-peracetyl-β-glucose:
[0058] The bromo 2,3,4,6-acetyl-β-glucose (1.3g, 3.85mmol) obtained by evaporating to dryness was dissolved in 5mL DMF, and sodium azide (500mg, 7.69mmol) was slowly added under ice-bath conditions, The reaction was terminated after 12 h at room temperature. After the reaction was completed, 10 mL of water was added to quench the reaction, and then the organic phase was extracted with chloroform (3×10 mL). After the organic phase was combined, dried with anhydrous sodium sulfate, concentrated under reduced pressure to obtain a crude product, which was purified by silica gel chromatography (elution Condition: petroleum ether / ethyl acetate=2:1). The product azide-2,3,4,6-acetyl-β-glucose 679.5 mg was obtained, and the yield was 52%. 1 H-NMR (CDCl 3 ,500MHz)δ5.20(t,1H,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com