Chiral drug mass spectrometry quantitative analysis method based on chemical derivatization reaction and spectral deformation quantitative analysis theory
A technology for quantitative analysis and chiral drugs, applied in the field of mass spectrometry quantitative analysis of chiral drugs, can solve the problems that quantitative analysis results are susceptible to experimental errors, background interference, etc., and achieve the effects of wide application range, simple use, and cost saving
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
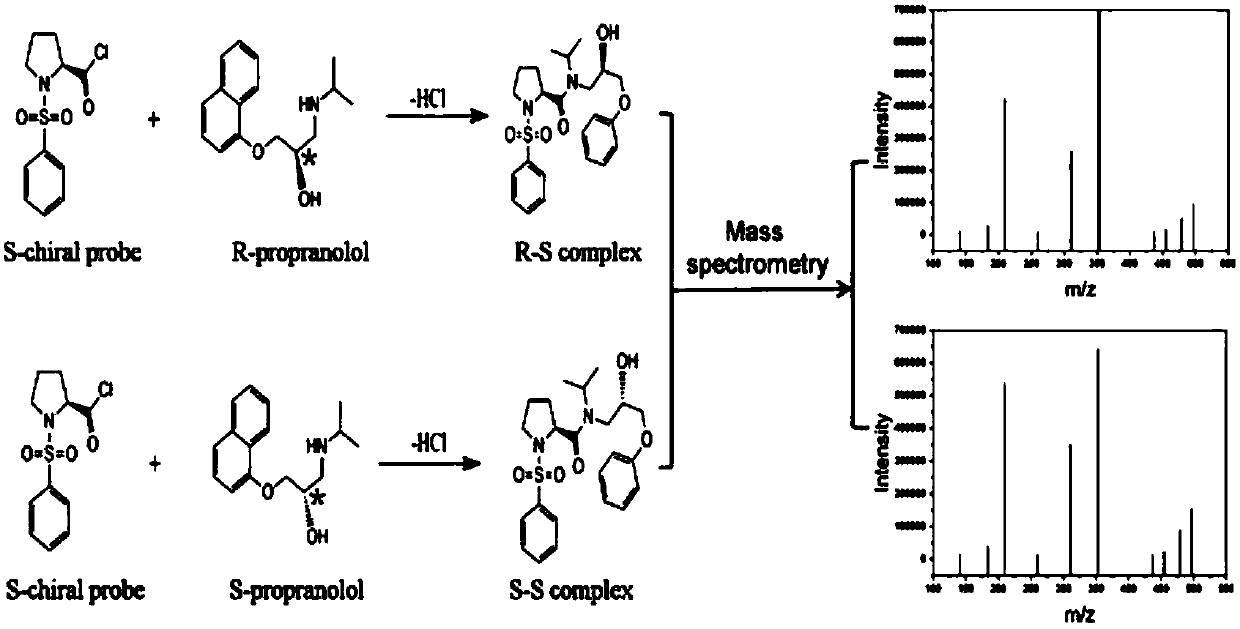
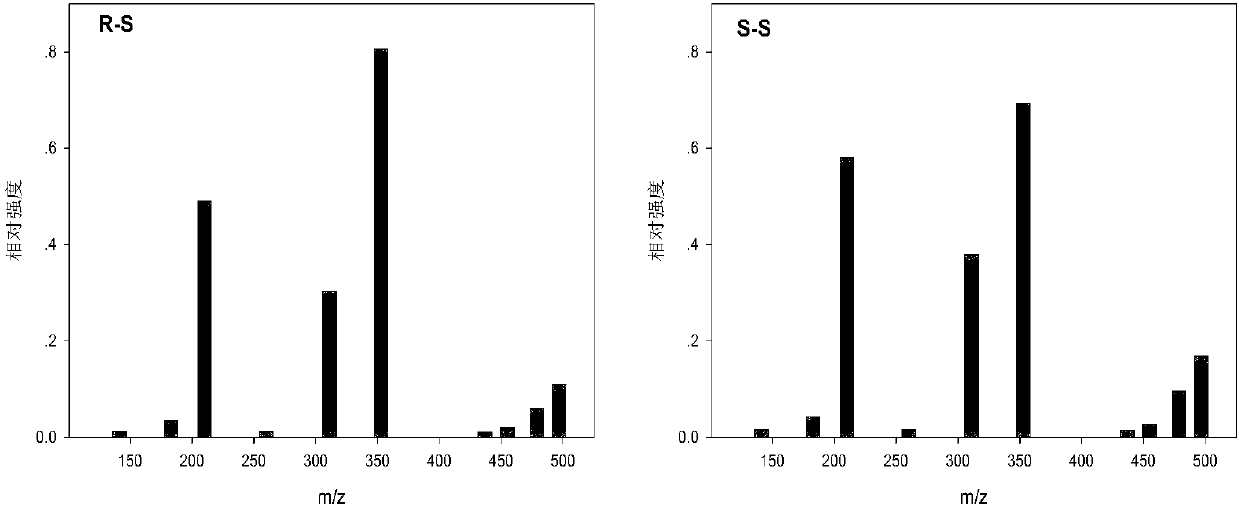
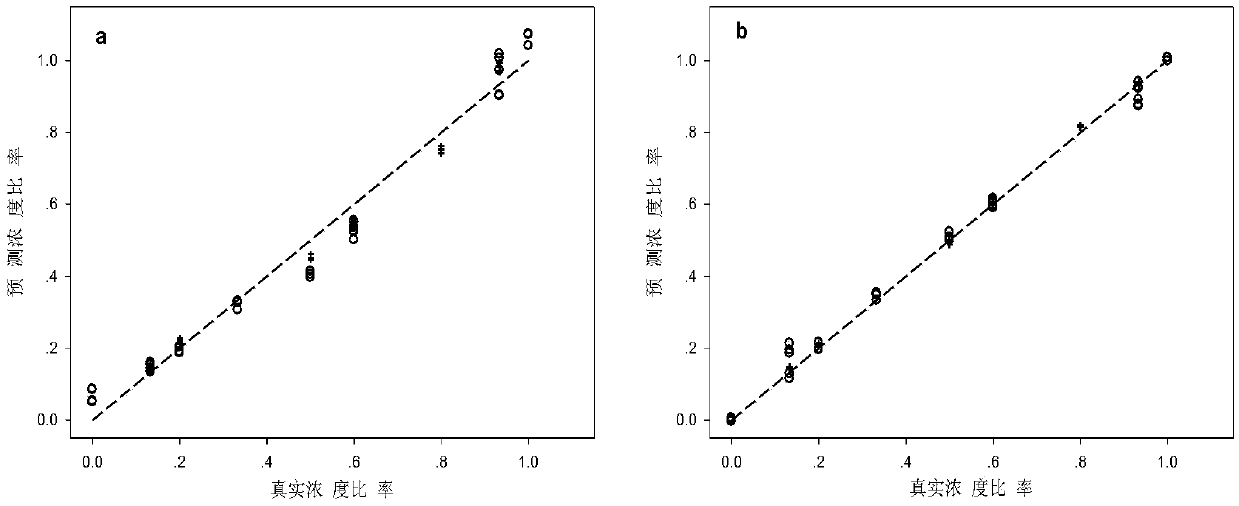
[0039] Quantitative detection of chiral propranolol in tablets by mass spectrometry based on chemical derivatization reaction and spectral deformation quantitative analysis theory
[0040] Propranolol (propranolol), as a traditional β-adrenoceptor blocker, is widely used in clinical treatment of arrhythmia and antihypertension. Propranolol has two optical isomers, R-type and S-type. These two enantiomers are mainly metabolized by cytochrome P450 in vivo, and there are differences in stereoselectivity. Animal experiments have shown that the S-enantiomer β-receptor blocking effect is about 100 times stronger than that of the R-enantiomer, and has a longer half-life in the blood. At the same time, R-propranolol has the function of suppressing libido and is a male contraceptive. Propranolol has always been administered in the form of racemate in clinical practice. Since the activities of the two isomers are very different, it is of great significance to develop a simple and prac...
PUM
| Property | Measurement | Unit |
|---|---|---|
| recovery rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com