Establishment of self-assembly nanoparticles of redox hypersensitive disulfide bond bridged prodrug
A technology of self-assembled nanoparticles and disulfide bridges, which is applied in drug combinations, pharmaceutical formulations, anti-tumor drugs, etc., can solve problems such as poor stability, low solubility, and large toxic and side effects, and achieve good stability and simple preparation process , Easy surface modification effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
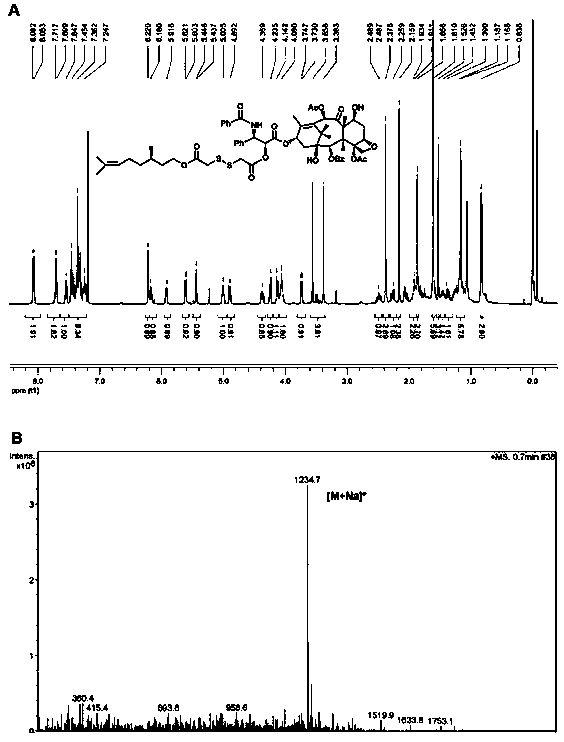
[0041] Example 1: Synthesis of a small-molecule prodrug of paclitaxel-citronellol (α-PTX-SS-CIT) with a disulfide bond at the α position of the carbonyl group
[0042] Add an appropriate amount of 2,2'-dithiodiacetic acid to a 50mL round bottom flask and dissolve it with 3mL of acetic anhydride. Stir at room temperature for 2 hours. Monitor the reaction process by thin layer chromatography. Then add 20mL of toluene into the system in three portions In and dry under reduced pressure distillation. The obtained product was dissolved in 30 mL of dichloromethane, and an appropriate amount of citronellol and DMAP was added, and stirred at room temperature for 1 hour. The reaction process was monitored by thin layer chromatography, and the intermediate product was purified by silica gel column chromatography. Finally, the intermediate product, EDCI, HOBt, and DMAP were dissolved in 50 mL of anhydrous dichloromethane, ice bathed for 1 hour, then an appropriate amount of paclitaxel was ad...
Embodiment 2
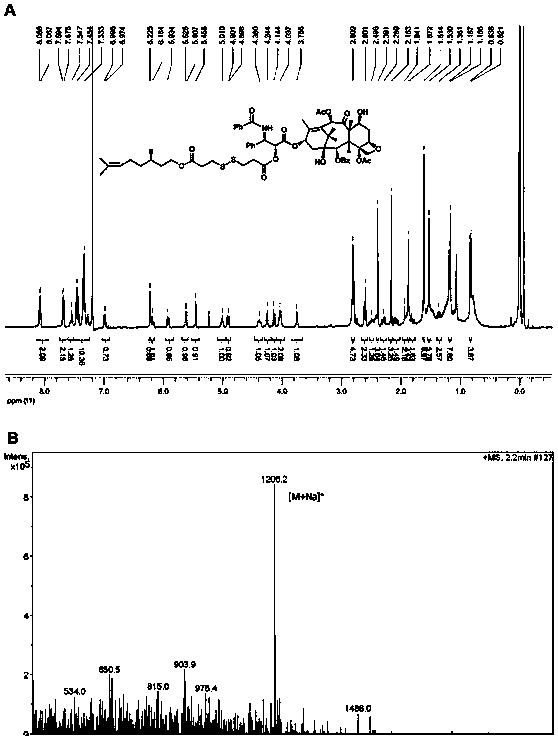
[0045] Example 2: Synthesis of a small-molecule prodrug of paclitaxel-citronellol (β-PTX-SS-CIT) with a disulfide bond at the β position of the carbonyl group
[0046] Add an appropriate amount of 3,3'-dithiodipropionic acid to a 50mL round bottom flask, and dissolve it with 3mL of acetic anhydride, stir at room temperature for 2 hours, monitor the reaction process by thin layer chromatography, and then add 20mL of toluene in three portions In the system, and carry out vacuum distillation and drying. The obtained product was dissolved in 30 mL of dichloromethane, and an appropriate amount of citronellol and DMAP was added, and stirred at room temperature for 1 hour. The reaction process was monitored by thin layer chromatography, and the intermediate product was purified by silica gel column chromatography. Finally, the intermediate product, EDCI, HOBt, and DMAP were dissolved in 50 mL of anhydrous dichloromethane, ice bathed for 1 hour, then an appropriate amount of paclitaxel w...
Embodiment 3
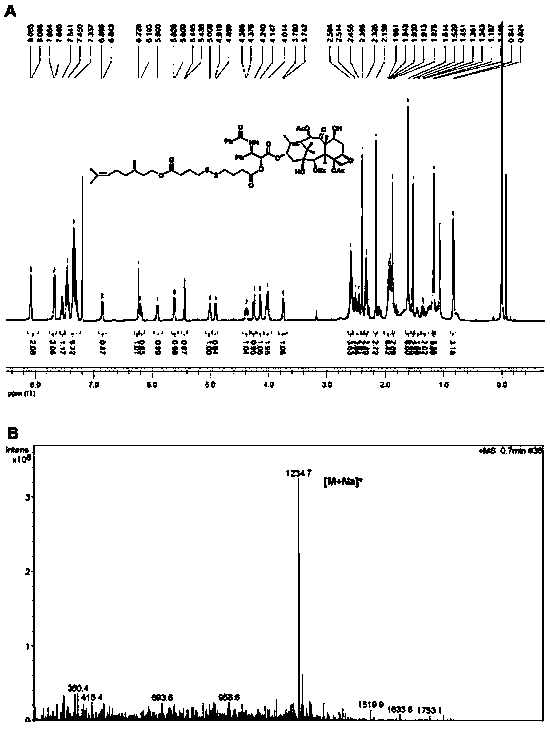
[0049] Example 3: Synthesis of a small-molecule prodrug of paclitaxel-citronellol (γ-PTX-SS-CIT) with a disulfide bond at the γ position of the carbonyl group
[0050] Add an appropriate amount of 4,4'-dithiodibutyric acid to a 50mL round bottom flask and dissolve it with 3mL of acetic anhydride. Stir for 2 hours at room temperature. Monitor the reaction process by thin layer chromatography. Then add 20mL of toluene in three portions In the system, and carry out vacuum distillation and drying. The obtained product was dissolved in 30 mL of dichloromethane, and an appropriate amount of citronellol and DMAP was added, and stirred at room temperature for 1 hour. The reaction process was monitored by thin layer chromatography, and the intermediate product was purified by silica gel column chromatography. Finally, the intermediate product, EDCI, HOBt, and DMAP were dissolved in 50 mL of anhydrous dichloromethane, ice bathed for 1 hour, then an appropriate amount of paclitaxel was adde...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com