Preparation method and application of rhenium disulfide nanoflake
A rhenium disulfide and nanosheet technology, which is applied in the field of preparation of single-layer and/or few-layer rhenium disulfide nanosheets, can solve the problems of high cost and harsh reaction conditions, and achieve low cost, high reactivity and low price Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] 0.5000g ammonium rhenate ((NH 4 ) 2 ReO 4 ) and 0.5400g sodium ethyl xanthate (C 3 h 5 OS 2 Na) were dissolved in 30mL deionized water respectively, and after being completely dissolved, the (NH 4 ) 2 ReO 4 The pH of the solution was adjusted to 7.00, after which the C 3 h 5 OS 2Na solution was added to it and mixed evenly. The mixture was transferred to a 100mL autoclave, sealed, placed in an oven at 200°C for 8 hours, and cooled to room temperature naturally. After the reaction product is separated, it is washed with deionized water and absolute ethanol several times, collected and vacuum freeze-dried to obtain a black powder product, which is rhenium disulfide material. Among them, ammonium rhenate is almost quantitatively converted to rhenium disulfide.
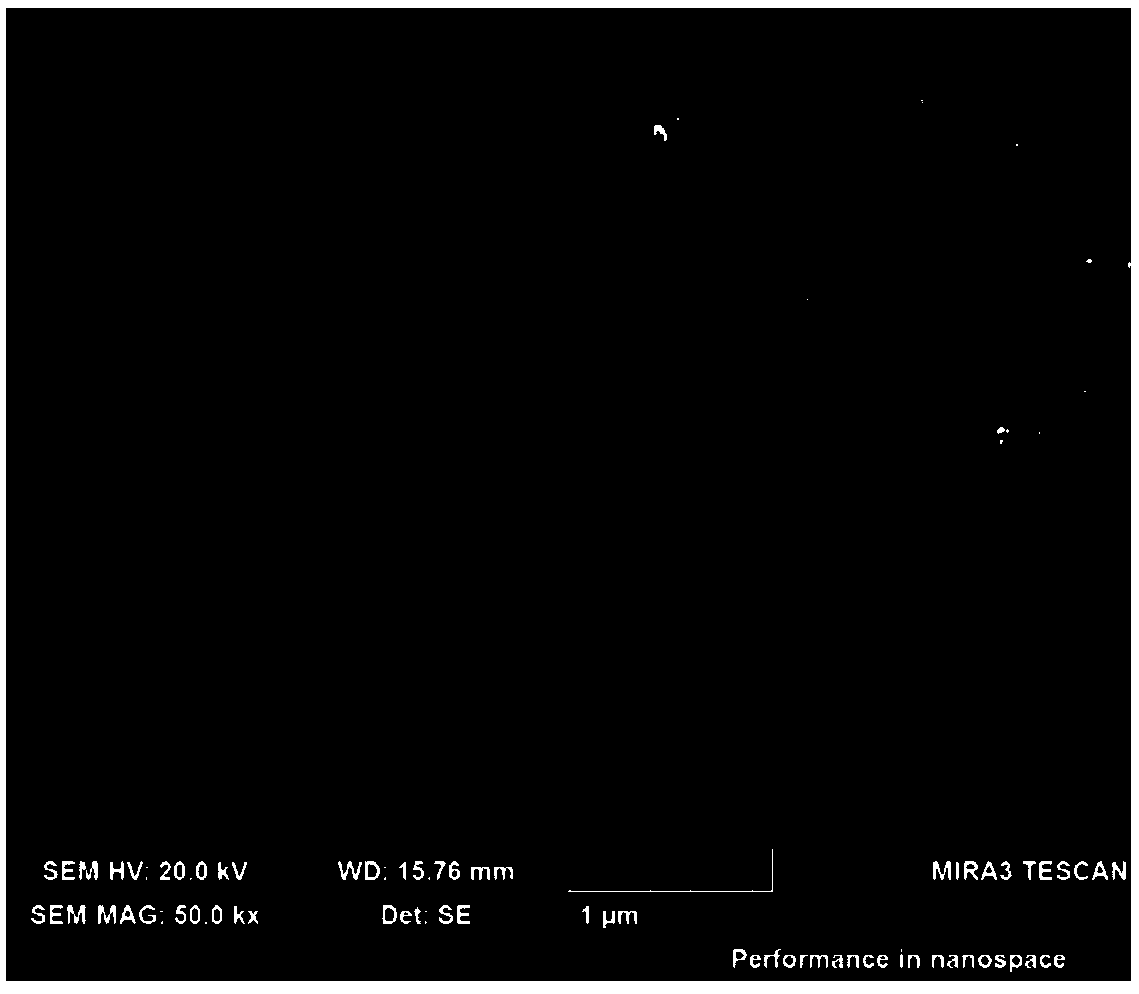
[0047] figure 1 The field emission scanning electron microscope (FESEM) picture of the rhenium disulfide material prepared for this embodiment, from figure 1 It can be seen that it is composed of many...
Embodiment 2
[0049] 0.5000g ammonium rhenate ((NH 4 ) 2 ReO 4 ) and 0.5400g sodium ethyl xanthate (C 3 h 5 OS 2 Na) were dissolved in 30mL deionized water respectively, and after being completely dissolved, C 3 h 5 OS 2 Na solution was added (NH 4 ) 2 ReO 4 solution, mix the two evenly. The mixture was transferred to a 100mL autoclave, sealed, placed in an oven at 200°C for 10h, and cooled to room temperature naturally. After the reaction product is separated, it is washed with deionized water and absolute ethanol several times, collected and vacuum freeze-dried to obtain a black powder product, which is rhenium disulfide material. Among them, ammonium rhenate is almost quantitatively converted to rhenium disulfide.
Embodiment 3
[0051] 0.5000g ammonium rhenate ((NH 4 ) 2 ReO 4 ) and 0.5400g sodium ethyl xanthate (C 3 h 5 OS 2 Na) were dissolved in water respectively, and after being completely dissolved, the (NH 4 ) 2 ReO 4 The pH of the solution was adjusted to 9.00, after which the C 3 h 5 OS 2 Na solution was added to it and mixed evenly. The mixture was transferred to a 100mL autoclave, sealed, placed in an oven at 190°C for 12h, and cooled to room temperature naturally. After the reaction product is separated, it is washed with deionized water and absolute ethanol several times, collected and vacuum freeze-dried to obtain a black powder product, which is rhenium disulfide material. Among them, ammonium rhenate is almost quantitatively converted to rhenium disulfide.
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com